1349
First results from an MRI compatible small animal PET insert operating in a clinical 3T PET/MRI1Imaging Program, Lawson Health Research Institute, London, ON, Canada, 2Department of Medical Biophysics, University of Western Ontario, London, ON, Canada, 3Cubresa, Winnipeg, MB, Canada, 4National Research Council of Canada, Winnipeg, MB, Canada, 5Department of Radiology, University of Manitoba, Winnipeg, MB, Canada, 6Department of Physics and Astronomy, University of Manitoba, 7PET/CT Imaging Program, Section of Nuclear Medicine, Health Sciences Centre, Winnipeg, MB, Canada
Synopsis
Reaching sub-millimeter limits in spatial resolution and improved temporal resolution, simultaneous PET/MRI using a pre-clinical MR compatible PET insert enables new discoveries in science and medicine. We recently evaluated a versatile MR compatible small animal PET insert in collaboration with Cubresa. Presented here are the first simultaneous images obtained using both imaging modes within our clinical 3T MRI.
Introduction
There is a need for cutting edge pre-clinical molecular imaging that exceeds the limitations in spatial and temporal resolution of clinical systems and enables new pathways for discovery in science and medicine. Though already proven powerful on their own, the fusion of pre-clinical positron emission tomography (PET) and magnetic resonance imaging (MRI) will provide new ways to image structure and function1,2. We recently evaluated a versatile MR compatible small animal PET insert in collaboration with Cubresa to enable true simultaneous pre-clinical PET/MRI studies. Presented here are the first simultaneous images obtained using both imaging modes in our 3T clinical scanner.
Methods
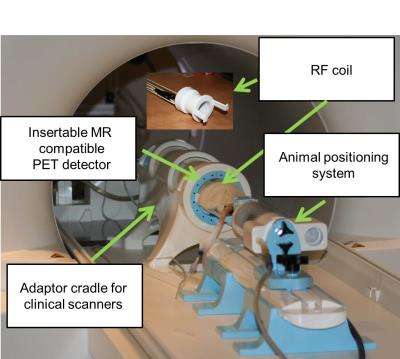
Animal experiments were approved by the local animal ethics committee and followed the guidelines set out by the CCAC while isotope usage was governed by a valid CNSC license. One male Sprague Dawley rat (m = 472g) was anesthetized using a mixture of 2% isoflurane with oxygen, catheterized via the tail vein and placed on an animal positioning table complete with nose cone (Fig 1). An insertable MR compatible PET system (Cubresa) was positioned inside a clinical 3T PET/MRI system (Siemens Biograph mMR) together with a head-only RF coil tuned to the 1H frequency (Fig 1). PET imaging was performed during two 30 min intervals following a bolus of 18F-FDG with an initial activity of 26.6 MBq. Images were reconstructed using a maximum expectation likelihood maximization (MLEM) algorithm with a reconstructed spatial resolution of 0.64 x 0.64 x 0.64 mm3 isotropic. T2-weighted MR images were acquired using the following parameters: FOV 70 x 70 mm, 0.22 x 0.22 mm2 in-plane resolution, 1.5 mm slice thickness with interslice spacing of 1.65 mm, TR/TE 3250/97 ms and bandwidth of 223 Hz/px. Additional sequences acquired during PET acquisition included 3D T1-weighted volumetric interpolated brain examination (VIBE) with 0.3 x 0.3 x 0.3 mm3 isotropic resolution, 3D T2-weighted SPACE (0.5 x 0.5 x 0.5 mm3 isotropic resolution) as well as T1 and T2 mapping sequences spanning the brain. PET and MR acquisition was simultaneous.Results
Figure 2A demonstrates our ability to acquire high resolution T2-weighted images of the rat head using a clinical 3T MRI and rat head RF coil. Both sagittal and axial T2-weighted images were acquired in 13 minutes simultaneously with the PET acquisition. No image artifacts due to the PET insert were observed. Figure 2B demonstrates fusion of the PET data with the T2-weighted images demonstrating good 18F-FDG sensitivity and co-localization within the brain. No performance issues were observed during the PET acquisition while the MRI gradient coils were active.Discussion
We present our first experience using an MR compatible small animal PET insert within a clinical 3T MR imaging system. Initial experience and image results demonstrate that there does not appear to be any deleterious effect of each system on the other. Future work includes rigorous characterization of each system both during simultaneous operation and during individual operation in order to characterize B0 and B1 homogeneity as well as the PET systems’ spatial resolution, scatter fraction performance and detector sensitivity as set out by NEMA standards NU 4-20083. Simultaneous PET/MRI will enable new pathways for scientific discovery in molecular imaging and medicine.Acknowledgements
The authors would like to acknowledge Lise Desjardins, Lynn Keenliside, Jennifer Hadway and Reggie Taylor for their help in animal care, prototyping and sequence development. Funding sources include NSERC Discovery, Ontario Research Fund - Research Excellence Round 7 and support from MITACS Accelerate for MSF.References
1. Goertzen, A. L., Stortz, G., Thiessen, J. D., Bishop, D., Khan, S., Kozlowski, P., ... & Thompson, C. J. (2016). First results from a high—resolution small animal SiPM PET insert for PET/MR imaging at 7 T. IEEE Trans. Nucl. Sci.
2. Weissler, B., Gebhardt, P., Dueppenbecker, P. M., Wehner, J., Schug, D., Lerche, C. W., ... & Perkuhn, M. (2015). A digital preclinical PET/MRI insert and initial results. IEEE transactions on medical imaging, 34(11), 2258-2270.
3. National Electrical Manufacturers Association (NEMA). NEMA Standards Publication NU 4-2008: Performance Measurement for Small Animal Positron Emission Tomographs. Washington. DC: NEMA;
Figures