1342
Application of MR-based truncation correction (HUGE) in whole-body PET/MR hybrid imaging1University Hospital Essen, Essen, Germany, 2Siemens Healthcare GmbH, 3Erwin L. Hahn Institute for Magnetic Resonance Imaging
Synopsis
In quantitative PET-imaging, it is essential to correct for the attenuation of photons in tissue. In combined PET/MR-imaging the attenuation correction (AC) is based on MR-data and subsequent tissue class segmentation. The MR-FOV is limited due to B0-inhomogeneities and gradient nonlinearities. Therefore, the AC-map is truncated and reconstructed PET-data are biased. HUGE (B0-Homogenization using gradient enhancement), which determines an optimal readout gradient to compensate gradient nonlinearities, was applied in whole-body PET/MR examinations of 24 oncologic patients. The extension of the MR-FOV for MR-based AC showed an improvement of PET-quantification in integrated PET/MR-imaging by reducing the truncated areas of the AC-map.
Purpose
To achieve quantitative PET imaging, it is essential to correct for the attenuation of photons in human tissue. In combined PET/MR hybrid imaging the attenuation correction (AC) is based on MR data and subsequent tissue class segmentation [1]. In MR imaging, the field-of-view (FOV) is limited to a diameter of typically 50 cm in x-y direction due to B0 inhomogeneities and gradient nonlinearities. Therefore, the AC map (μmap) might be truncated or geometrically distorted at the edges of the FOV, and hence the reconstructed PET data may be biased. In this work, a method called HUGE [2] (B0 homogenization using gradient enhancement), which determines an optimal readout gradient in x-direction to locally compensate the gradient nonlinearities, was applied in whole-body PET/MR examination of 24 oncologic patients to quantify the impact of truncation correction on PET data.Methods
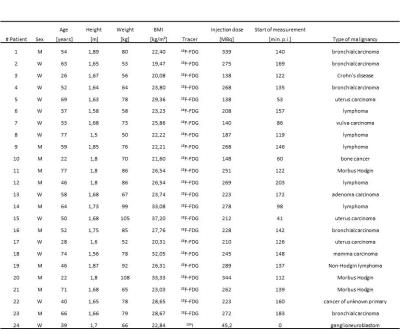
All measurements were performed on an integrated whole-body PET/MR system (Biograph mMR, Siemens Healthcare, Erlangen, Germany). Within this study, 24 patients (mean age ± SD, 50.9 ± 17.4 y; age range 22-77 y; 13 female and 11 male) underwent a clinical indicated PET/CT examination and a subsequent whole-body PET/MR acquisition. Detailed patient information are listed in Tab.1. For AC map processing, the acquisition protocol consists of a standard Dixon-VIBE sequence (image matrix 192x126x128, resolution 2.6x2.6x3.1 mm³, TE 2.46 ms, TR 3.6 ms, FA 10°) serving as reference standard. A modified HASTE sequence with continuous table movement (image matrix 128x128, resolution 2.3x2.3 mm², TE 23 ms, TR 612 ms, FA 120°) that was acquired to obtain MR signal from the extended FOV (HUGE) [3]. The HUGE MR data is used to complete the truncated Dixon-AC map by filling the missing parts with a linear attenuation coefficient of 0.1 cm-1 (Fig.1). All PET reconstructions were performed with e7 tools (Siemens Molecular Imaging, Knoxville, USA) with OP-OSEM algorithm, 3 iterations, 21 subsets and 4 mm Gaussian filter. To evaluate the impact of truncation correction on PET data all patient data were reconstructed three times: Dixon-, HUGE- and MLAA-μmap. Volumes-of-interest (VOIs) were identified and analyzed with regard to standard uptake value (SUV). Difference images of patient data were calculated.Results
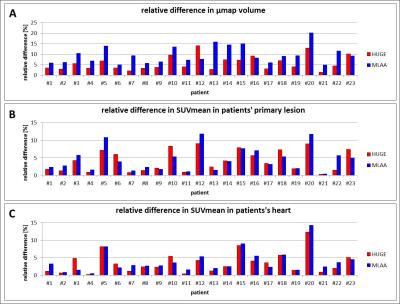
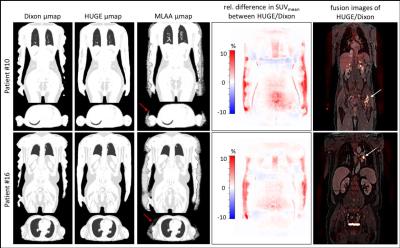
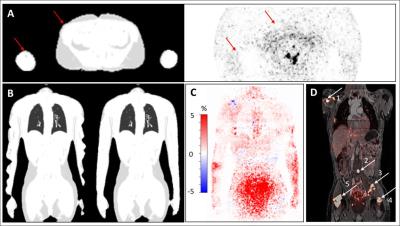
The average increase in μmap volume using HUGE is 5.78 ± 3.38 %, for MLAA 9.65 ± 4.00 % compared to standard Dixon AC (Fig.1). The average increase in SUVmean in each patient’s primary lesion using HUGE is 4.17 ± 2.96% and for MLAA 4.57 ± 3.30 % (Fig.1). Maximal relative differences in SUV occur in patients with lesions close to truncations (#10 with 9.61 %, #12 with 14.05 % for HUGE) or in patients with high BMI (#15 with 7.21 %, #20 with 12.98 % for HUGE). Fig.2 depicts the improvement of extended AC map. While the segmented μmap of the Dixon-VIBE sequence shows signal truncation along the arms, HUGE MR-data add body volume along patient’s arms. Relative difference images in PET signal of two patients (Fig.2) show that the highest impact of HUGE arises in the thorax region and in the added volume parts of patient’s arms. Fig.3 shows the results of the non-FDG patient #24, who was measured with I-124. Contour detecting of the patient’s body with PET is limited in this example (e.g. MLAA [4]), but HUGE, as a fully MR-based approach, is independent of the choice of radiotracer. The gain in μmap volume using extended AC-map increases to 5.67 % compared to standard Dixon-VIBE imaging. The highest impact in percentage deviation in SUVmean is observable in lesion #4 (14.01 %), which is close to truncations. Minimal increase of SUVmean occurs in lesion #1 (0.85 %).Discussion and Conclusion
In patient measurements the extension of the MR FOV using HUGE for MR-based truncation correction showed an improvement of PET quantification in integrated PET/MR imaging by reducing the otherwise truncated areas of the patient μmap. The impact of HUGE highly depends on the position of the patient’s lesion relative to regions where truncations appear. Despite the variance in PET quantification from patient to patient, the proposed method HUGE depicts that a truncation correction for a broad range of patients with different lesions is needed to further improve PET quantification in integrated PET/MR hybrid imaging. HUGE as a fully MR-based approach is independent of the PET radiotracer choice, and thus, provides reliable accurate truncation correction in non-FDG patients, where other methods of PET contour detection might have failed. HUGE combined with CTM is a robust method and now allows for its implementation on integrated PET/MR systems.Acknowledgements
No acknowledgement found.References
1) Martinez-Möller A, Souvatzoglou M, Delso G, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009; 50(4):520-526
2) Blumhagen JO, Ladebeck R, Fenchel M, et al. MR-based field-of-view extension in MR/PET: B0 homogenization using gradient enhancement (HUGE). Magn Reson Med. 2013; 70(4):1047-1057
3) Blumhagen JO, Braun H, Ladebeck R, et al. Field of view extension and truncation correction for MR-based human attenuation correction in simultaneous MR/PET imaging. Med Phys. 2014; 41(2):022303. doi:10.1118/1.4861097
4) Nuyts J, Michel C, Fenchel M, et al. Completion of a truncated attenuation image from the attenuated PET emission data. IEEE Nucl Sci Symp Conf Record. 2010; 2123–2127.
Figures