1340
Improved clinical workflow for simultaneous whole-body PET/MRI using high-resolution CAIPIRINHA-accelerated MR-based attenuation correction1Department of Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Siemens Healthineers, Erlangen, Germany, 3Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Divison of Radiopharmaceutical Chemistry, German Cancer Research Center (DKFZ), Germany, 5Clinical Cooperation Unit Nuclear Medicine, German Cancer Research Center (DKFZ), Heidelberg, Germany, 6Nuclear Medicine, University Hospital Heidelberg, Heidelberg, Germany, 7Junior Group Medical Image Computing, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
The present study assesses the value and reproducibility of a novel CAIPIRINHA-accelerated T1-weighted Dixon-based prototype for whole-body PET/MRI in comparison to the clinical standard. This prototype allows the aquisition of an MR-based attenuation correction and a high-resolution T1w DIXON stack that may be used for diagnostic correlation of PET findings in one single step. Voxel-wise intra-individual differences, intermethod-agreement using regression and bland-altman plot analysis, inter-reader agreement for image quality and a repeated measurement experiment in a healthy volunteer without tracer injection were peformed. The novel prototype demonstrated a high reproducibility of standardized uptake value quantification compared to the standard and excellent image quality for all body regions. Smaller breathing artifacts in the lungs may transfer on the PET µmap and thus influence the attenuation correction. Therefore, physicians and the technicians need to assess the µmap to veriy artifact-free acquisition. The novel protoype is useful for clinical PET/MRI studies towards time-efficient protocols as a separate T1w-sequence may be omitted.
Purpose
Attenuation correction (AC) in whole-body simultaneous positron emission tomography/magnetic resonance imaging (PET/MRI) is usually performed using a two-point DIXON T1w sequence [1] with reliable results compared to PET/CT demonstrated for more than 2300 patients [2]. However, the DIXON stacks acquired are only usuable for AC because its resolution in axial orientation (4.1 x 2.6 x 3.1 mm3) is not optimal for diagnostic assessment. The present study explores the value and reproducibility of a novel magnetic resonance based attenuation correction (MRAC) using a high-resolution (1.9 x 1.4 x 3.2 mm3) CAIPIRINHA-accelerated T1-weighted Dixon-based prototype (MRACcaipi) for PET/MRI compared to the clinical standard (MRACstd).Materials and Methods
All measurements were performed on a 3 Tesla Siemens Biograph mMR (Erlangen, Germany), capable of whole-body imaging. The study was approved by the institutional review board. A prototype CAIPIRINHA VIBE sequence implementation by Siemens Healthineers (Erlangen, Germany) was used allowing for higher parallel imaging acceleration factors on the MR side along with the CAIPIRINHA delta shift parameters to twist the undersampling pattern in the 2 phase encoding directions. The reconstruction pipeline includes inline Dixon fat/water separation as well as the 4-compartment MR-based attenuation correction segmentation algorithm adapted to higher resolution input images. The prototype sequence also supports inline reconstruction of PET AC images from parallel PET acquisitions. CAIPIRINHA-settings were: acceleration factor PE 2, acceleration factor 3D 2, delta shift PAR 1, ref lines PE 24, ref lines PAR, slice oversampling 40%, and 80 slices per slab. PET data were iteratively (3D) reconstructed using an ordered subsets expectation maximization (OSEM) algorithm with 3 iterations, 21 subsets and Gaussian post-filtering with a full-width at half-maximum 2.The PET raw data of 19 patients from clinical routine with various tracers were reconstructed with MRACstd and the novel CAIPIRINHA-accelerated MRACcaipi, both 19 s/bed position. Volume of interests (VOIs) for liver, lung and all voxels of the total image stack were created to calculate standardized uptake values (SUVmean) followed by inter-method agreement using Passing-Bablok regression and Bland-Altman plot. A voxel-wise SUV comparison per patient was performed for intra-individual correlation between MRACstd and MRACcaipi. Difference images of the attenuation maps (MRACstd-MRACcaipi) and SUV images were created. A healthy volunteer was scanned repeatedly for 5 times (no tracer injection) in one session over 7 bed-positions for whole-body assessment with both MRACstd and MRACcaipi. The image quality of MRACcaipi-derived sequences was assessed by two blinded board-certified radiologists on a Likert-scale (1-5) including intra-class coefficients for inter-reader agreementResults
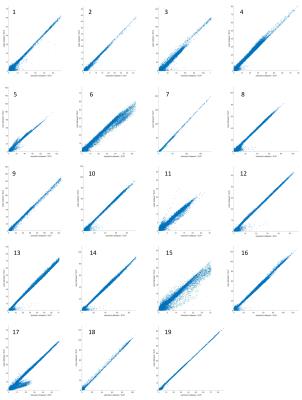
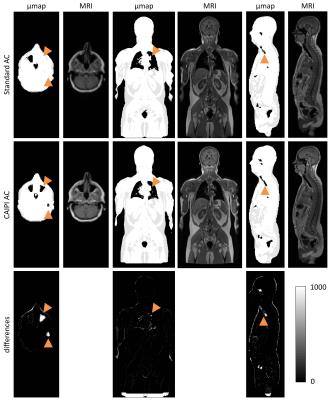
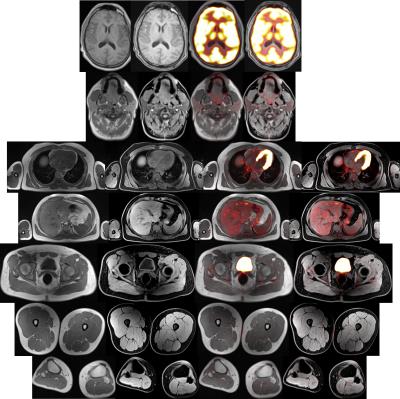
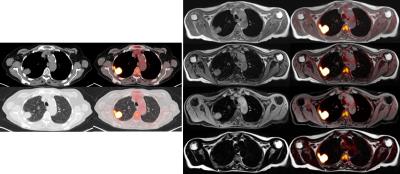
SUVmean correlations of VOIs were close to linearity with high significance (0.95<Spearman’s rho<1, p<0.0001, respectively). See Figure 1 for SUVmean comparison of all patients of the complete image stack. Scatter-plots of voxel-wise comparison per patient showed significant linearity (Figure 2, 1.79x108 pixels were evaluated in total). Points of deviation from the center line could be explained by different segmentation of air-containing tissue, lungs, kidneys, metal implants, diaphragm edge or small air bubbles in the gastrointestinal tracts that moved between MRAC acquisitions. The repeated-measurements experiment with one individual demonstrated small differences at the edges of the diaphragm (slightly different level of breath-hold positions in between the acquisitions) and air bubbles in the bowel that moved between the acquisition of MRACstd and MRACcaipi. Nasal sinuses and the trachea were better segmented in MRACcaipi (Figure 3). High-resolution T1w Dixon images were acquired in all cases and could be used for PET-fusion purposes (Figure 4,5). The diagnostic images derived from MRACcaipi were of high diagnostic quality (4.2±0.8) with 0.92-0.96 intra-class correlation.Discussion
The novel protoype MRACcaipi extends the value for attenuation correction in PET/MRI by providing a high-resolution image series in DIXON technique suited for diagnostic assessment. The whole setup is performed in the same amount of time as the clinical standard (19s/bed position). The SUV quantification resulting from the MR-based µmap was shown to be reproducible and robust. While some anatomical parts are better delineated in the new µmap from MRACcaipi, breathing artifacts may sometimes occur in the thorax that transfer on the µmap. These artifacts need to be checked by technician and physician in the µmap although artifact-free images may be easily acquired in compliant patients. The novel protoype is useful for clinical PET/MRI studies towards time-efficient protocols as a usually separately acquired T1w-sequence may be omitted.Acknowledgements
No acknowledgement found.References
[1] Martinez-Möller et al., Journal of Nuclear Medicine, 2009.
[2] Spick et al., Journal of Nuclear Medicine, 2016
Figures