1335
Hybrid ZTE/Dixon MR-based Attenuation Correction for Knee PET/MR Sodium Fluoride Studies1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2GE Global Research, Munich, Germany, 3GE Healthcare, Waukesha, WI, United States
Synopsis
This study introduces a new hybrid ZTE/Dixon MR-based attenuation correction (MRAC) method including bone density estimation for PET/MRI and quantifies the effect of bone attenuation on sodium fluoride uptake in the knee.
Introduction
Accurate PET standard uptake values (SUVs) estimation is important for multi-modality quantitative imaging studies. Currently commercially available MRAC methods for body PET imaging use a fat/water map derived from a “Dixon” MRI sequence, where only attenuation of soft-tissue fat and water components are considered and bone is neglected1. Zero echo-time (ZTE) MRI has demonstrated that the bone density in the head was found to be inversely proportional to the signal intensity2.
A hybrid MRAC method (“Hybrid ZTE/Dixon MRAC”3) through the combination of ZTE MRI (for bone segmentation and density estimation) and Dixon MRI (for water, fat, and air classification) was tested for knee PET/MRI.
Methodology
Sixteen patients (age 57±10 years) were scanned on a time-of-flight (TOF) PET/MRI scanner (GE Signa PET/MR) with (18F-NaF) sodium fluoride. MR imaging for attenuation correction consisted of two-point Dixon (FOV=500×500×312mm, resolution=1.95×1.95mm, slice thickness=5.2mm, slice spacing=2.6mm, TE=[1.15ms,2.3ms], TR=4.05ms, 3D GRE) and zero echo-time (ZTE) pulse sequences (FOV=140x140x192mm, resolution=1.094mm×1.094mm×1.2mm, BW±62.5kHz, FA=2°, 1.024ms readout duration).
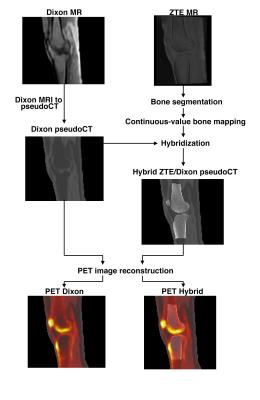
A continuous-value fat and water Dixon pseudoCT was generated from two-point Dixon MRI. Bone was segmented from the ZTE images and converted to Hounsfield units (HU) using a continuous two-segment piecewise linear model based on ZTE MRI intensity. The HU values were converted to linear attenuation coefficients (LAC) using a bilinear model. The bone voxels of the Dixon-based pseudoCT were replaced by the ZTE-derived bone to produce the hybrid ZTE/Dixon pseudoCT. Figure 1 displays the overall methodology.
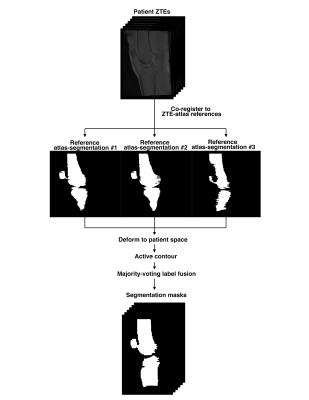
A multi atlas-based segmentation is introduced to automate the bone segmentation. A ZTE atlas was generated from the ZTE images of the three patients. Each patient ZTE was co-registered to each atlas-ZTE and the atlas-segmentation map was deformed into the patient space. This initial segmentation served as an initialization to an active contour segmentation method that yields the final segmentation map from each atlas-segmentation. A majority-voting label fusion strategy was used to merge the segmentation produced by the three references. Figure 2 displays the flowchart for the multi atlas-based automatic segmentation.
The three different AC maps (Dixon, hybrid ZTE/Dixon with manual segmentation, and hybrid ZTE/Dixon with automatic segmentation) were used to reconstruct PET images using a TOF ordered subsets expectation maximization algorithm with a point-spread function model (FOV=600mm, 2 iterations, 28 subsets, matrix size=192×192, 89 slices of 2.78mm thickness).
For data analysis, each PET image was registered to a reference atlas where different volumes-of-interest (VOI) are defined. Correlation plots and Bland-Altman plots were used to compare the different methods. A Friedman test with a Tukey post hoc test were used to compare the different MRAC methods within each VOI.
Results
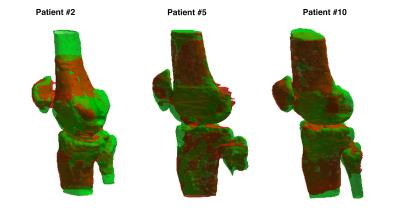
Manual bone segmentation and atlas-based automatic segmentation are compared in Figure 3. The automatic segmentation matches well with the manual segmentation with an average Jaccard index of 71.6%.
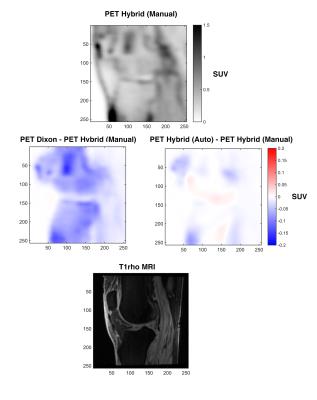
Sample PET and difference images are shown in Figure 4 and uptake within each VOI between methods are compared in Figure 5. There is large underestimation of uptake in and around bony regions with the Dixon method (no bone) compared to the hybrid methods (including bone); the difference across the VOIs is SUV and is a strong consistent bias (Spearman rho=0.9936, y=1.10x+0.02). Between the manual versus automatic segmentation for the hybrid method, there is a small difference in uptake and the difference across the VOIs is SUV with minimal bias (Spearman rho=0.9983, y=0.99x-0.01). The table in Figure 5 shows significant difference (p<0.05) between the Dixon-based and hybrid methods for each VOI.
Discussion
In our study, there is a significant difference in PET uptake in the volumes-of-interest when neglecting bone. These differences may affect the correlation of PET uptake to MRI parameters as well as comparisons between different patients and different systems. Our method includes ZTE MRI-based estimation of bone density to minimize these differences.
For this study, we only analyzed VOI-specific differences of SUV across all patients. It would be of interest to explore if there are any inter-patient or inter-VOI changes that result from the improved attenuation correction. Moreover, we did not have a gold-standard attenuation map (e.g. CT) for comparison, but the hybrid method with manual segmentation provides more realistic pseudoCTs compared to Dixon pseudoCTs without bone. To ultimately validate our method, we will collect subject CT data for comparison.
Conclusion
A hybrid pseudoCT (with fat and water soft tissue mapping, bone density estimation, and air segmentation) was successfully generated from combined ZTE and Dixon acquisitions for MR-based attenuation correction in PET/MRI. The Dixon-only pseudoCT significantly underestimates uptake in bone regions, indicating that bone cannot be ignored for accurate sodium fluoride uptake measurement.Acknowledgements
No acknowledgement found.References
1. Wollenweber SD, Ambwani S, Lonn AHR, et al. Comparison of 4-Class and Continuous Fat/Water Methods for Whole-Body, MR-Based PET Attenuation Correction. IEEE Trans Nucl Sci. 2013;60(5):3391-3398. doi:10.1109/TNS.2013.2278759.
2. Wiesinger F, Kaushik SS, Shanbhag DD, et al. ZT-AC: Zero TE based PET/MR attenuation correction. Int Soc Magn Reson Med 2016 24th Annu Meet Conf Proc. May 2016.
3. Leynes A, Yang J, Shanbhag DD, et al. Quantitative Evaluation of the Effect of Bone on Pelvic Lesion Uptake for MR-based Attenuation Correction on an Integrated Time-of-flight PET/MRI System. 24th Annu Meet Int Soc Magn Reson Med. May 2016.
Figures