1334
Zero-echo-time PET/MRI attenuation correction shows good correlation between 15O-water PET and simultaneously acquired ASL in standard regional flow territories1Surgical Sciences, Uppsala University, Uppsala, Sweden, 2Biomedical Technology, Medical Physics and IT, Uppsala University Hospital, Uppsala University Hospital, Uppsala, Sweden, 3Applied Science Laboratory, GE Healthcare, Uppsala, Sweden, 4Medicinal Chemistry, Uppsala University, Uppsala, Sweden, 5Neuroscience, Uppsala University, Uppsala, Sweden
Synopsis
Zero-Echo-Time (ZTE) MRI for attenuation correction (AC) in hybrid PET/MRI-systems is a promising method. This study aims to examine reproducibility between 15O-water PET and simultaneously acquired Arterial Spin Labelling (ASL) using ZTE-MRI AC and the reproducibility between to subsequent ASL measurements. Measurements were performed on six subjects on an integrated 3.0 T PET/MRI-system. Regional cerebral blood flow (CBF) values from standard flow territories were compared. ASL showed good correlation with 15O-water PET, presenting another advantage of ZTE-MRI AC. A significant decrease between ASL measurements was detected, which may be important to consider when designing PET/MRI-studies.
Introduction
Arterial spin labelling (ASL) magnetic resonance imaging (MRI) promises clinical value in several common neurological disorders, such as stroke, epilepsy and neurodegenerative decease, with the advantages of being quantifiable, non-invasive and cost efficient1, 2. Furthermore, cerebral blood flow (CBF) maps are reproducible and comparable to measurements with 15O-water position emission tomography (PET), the gold standard for CBF measurements3-8. However, only two studies have compared ASL to simultaneously measured PET in hybrid PET/MRI-systems6, 8. For optimal comparison, CBF measurements should be performed simultaneously, ideally with hybrid PET/MRI-systems. So far, accurate MR-based attenuation correction of PET data has been a challenge, compromising quantitative accuracy of PET/MRI-based brain-PET images9. Several AC-methods has been proposed; atlas-, Dixon segmentation- and ultra-short echo time (UTE) segmentation based methods are today available for clinical use10. These methods suffer from limitations such as lack of accuracy in the infratentorial regions, introducing significant bias in the cortical region and underestimation of bone 11-13. The use of Zero-Echo-Time (ZTE) MRI for AC has recently been suggested and shown promising results, and can provide accurate attenuation maps for quantitative brain studies10, 1.Purpose
This study aims to assess the reproducibility of ASL measurements in standard regional flow territories. And to compare CBF values from ASL with 15O-water PET derived-values, using ZTE-AC on a time-of-flight capable 3.0 T PET/MRI-system.Method
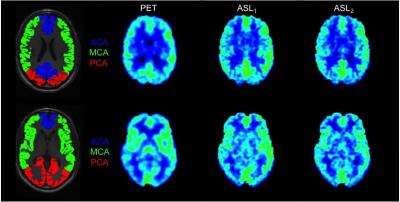
The patient group consisted of six subjects (four patients with epilepsy and two controls). All measurements were performed on a 3.0 T integrated PET/MRI-system (Signa PET/MR, GE Healthcare, Milwaukee, USA) using an 8-channel head coil (Invivo Hi-Res Head Coil, Gainesville, FL). All patients underwent a 10 min dynamic PET acquisition after automatic bolus injection of 5 MBq/kg 15O-water (1 ml/s during 5 s, followed by 35 ml saline at 2 ml/s). Blood was sampled continuously from a radial artery for measurement of an arterial input curve. Both during 15O-water PET and at about 2 h after (subject remained in the scanner), 3D FSE Pseudo-Continuous ASL (3D pCASL) with a spiral read-out was acquired and absolute quantifiable CBF maps were generated15-17. A sagittal high-resolution 3D-T1w image was acquired as structural reference and for segmentation. 15O-water PET images were reconstructed using time-of-flight OSEM with ZTE-based attenuation correction and a 5 mm post-filter, into 22 frames of increasing durations. Blood sampler data were corrected for delay and dispersion and CBF images were computed using a basis function implementation of the standard single-tissue compartment model including a fitted blood volume parameter. The 3D-T1w images were segmented into grey- and white matter tissue probability maps, normalised to MNI template space and 15O-water PET and ASL CBF maps were registered to each patients 3D-T1w image using SPM12 (Statistical Parameter Mapping toolbox, FIL, London, UK). Regions of anterior, middle and posterior flow territories were created from a standard vascular territory template in MNI space18 and was inversely transformed to each patient (figure 1). Flow regions were divided in left and right hemisphere, yielding a total of 6 flow regions. All flow regions were masked with each patients corresponding grey matter tissue map (50% probability) to exclude white matter contamination. Regional correlations between 15O-water PET and simultaneously acquired ASL1 were tested with Pearson product-moment correlation coefficient and difference between the first and second ASL acquisition was tested with a paired t-test.Results
Regional CBF values were significantly reduced between ASL1 and ASL2 for all flow regions (p < 0.01), mean relative decrease were 10%. There were significant correlations between 15O-water PET and ASL1 for all flow regions and total GM and WM respectively. Result of the 15O-water PET and ASL1 comparison is shown in table 1.Discussion
In this study we demonstrate good correlations with 15O-water PET and ASL measurements in regional flow territories as well as total grey- and white matter, comparable to other reports, using a hybrid 3T PET/MRI system with ZTE-segmentation attenuation correction8, 9. A significant decrease in regional CBF was observed for the subsequent ASL during the same scan session compared to the first ASL acquisition.Conclusion
ZTE segmentation is a promising method for attenuation correction of 15O-water PET data which correlates well with simultaneously acquire ASL data. It is warranted to consider a possible decrease in CBF measured by ASL during long scanning sessions when designing a study. The use of regional flow territories can have potential clinical applications in patients with dementia19 or cerebrovascular diseases effecting blood flow, for example moyamoya.Acknowledgements
No acknowledgement found.References
1. Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2015;73(1):102-16.
2. Mutsaerts HJ, van Osch MJ, Zelaya FO, Wang DJ, Nordhoy W, Wang Y, et al. Multi-vendor reliability of arterial spin labeling perfusion MRI using a near-identical sequence: implications for multi-center studies. Neuroimage. 2015;113:143-52.
3. Bokkers RP, Bremmer JP, van Berckel BN, Lammertsma AA, Hendrikse J, Pluim JP, et al. Arterial spin labeling perfusion MRI at multiple delay times: a correlative study with H(2)(15)O positron emission tomography in patients with symptomatic carotid artery occlusion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30(1):222-9.
4. Heijtel DF, Mutsaerts HJ, Bakker E, Schober P, Stevens MF, Petersen ET, et al. Accuracy and precision of pseudo-continuous arterial spin labeling perfusion during baseline and hypercapnia: a head-to-head comparison with (1)(5)O H(2)O positron emission tomography. Neuroimage. 2014;92:182-92.
5. Petersen ET, Mouridsen K, Golay X, all named co-authors of the Qt-rs. The QUASAR reproducibility study, Part II: Results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage. 2010;49(1):104-13.
6. Su Y, Vlassenko A, Blazey T, Ances B, Snyder A, Priatna A, et al. Comparison of cerebral blood flow measurement obtained from simultaneously acquired ASL and O15-water PET. Journal of Nuclear Medicine. 2014;55(supplement 1):207-.
7. Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer's disease. NMR in biomedicine. 2010;23(3):286-93.
8. Zhang K, Herzog H, Mauler J, Filss C, Okell TW, Kops ER, et al. Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(8):1373-80.
9. Fan AP, Jahanian H, Holdsworth SJ, Zaharchuk G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36(5):842-61.
10. Sekine T, Ter Voert EE, Warnock G, Buck A, Huellner MW, Veit-Haibach P, et al. Clinical evaluation of ZTE attenuation correction for brain FDG-PET/MR imaging-comparison with atlas attenuation correction. J Nucl Med. 2016.
11. Sekine T, Buck A, Delso G, Ter Voert EE, Huellner M, Veit-Haibach P, et al. Evaluation of Atlas-Based Attenuation Correction for Integrated PET/MR in Human Brain: Application of a Head Atlas and Comparison to True CT-Based Attenuation Correction. J Nucl Med. 2016;57(2):215-20.
12. Andersen FL, Ladefoged CN, Beyer T, Keller SH, Hansen AE, Hojgaard L, et al. Combined PET/MR imaging in neurology: MR-based attenuation correction implies a strong spatial bias when ignoring bone. Neuroimage. 2014;84:206-16.
13. Aasheim LB, Karlberg A, Goa PE, Haberg A, Sorhaug S, Fagerli UM, et al. PET/MR brain imaging: evaluation of clinical UTE-based attenuation correction. Eur J Nucl Med Mol Imaging. 2015;42(9):1439-46.
14. Sousa JM, Appel L, Papadimitriou S, Nyholm D, Sörensen J, Danfors T, et al. Validation of Zero-Echo Time PET-MR against stand-alone PET using dynamic dopamine transporter imaging. Eur J Nucl Med Mol Imaging. 2016;43(Suppl 1):S79.
15. Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magnetic resonance in medicine. 2012;67(5):1252-65.
16. Dai W, Shankaranarayanan A, Alsop DC. Volumetric measurement of perfusion and arterial transit delay using hadamard encoded continuous arterial spin labeling. Magnetic resonance in medicine. 2013;69(4):1014-22.
17. Maleki N, Dai W, Alsop DC. Optimization of background suppression for arterial spin labeling perfusion imaging. Magnetic Resonance Materials in Physics, Biology and Medicine. 2012;25(2):127-33.
18. Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50(6):1699-708.
19. Mutsaerts HJ, van Dalen JW, Heijtel DF, Groot PF, Majoie CB, Petersen ET, et al. Cerebral Perfusion Measurements in Elderly with Hypertension Using Arterial Spin Labeling. PloS one. 2015;10(8):e0133717.
Figures