1332
Water and Fat Based Partial Volume Correction for PET/MRI1Radiology, University of Wisconsin, MADISON, WI, United States, 2Electrical Engineering, University of Wisconsin, MADISON, WI, United States, 3University of Wisconsin, MADISON, WI, United States
Synopsis
In this study we propose to develop methods that improve the resolution of PET images by utilizing water and fat-based partial volume correction. These methods are expected to be particularly useful in simultaneous breast PET/MR imaging as white adipose tissue is known to be minimally FDG avid.
Introduction
While the fundamental resolution of PET is related to the annihilation distance of the positron, practical limitations are described by the size of the PET detectors and thus crystals. Due to practical tradeoffs between size, cost, and sensitivity, current PET scanners are able to achieve spatial resolution on the order 4 to 5 mm. When paired with an MR scanner, which can readily achieve spatial resolution of 1x1x1 mm in regions such as the head and the breast, the PET resolution is greatly mismatched. However, given the exquisite soft tissue contrast of MRI, PET images could be improved by making realistic assumptions based on anatomical properties obtained from the MR images. For example, white adipose tissue does not utilize significant amounts of glucose (for which FDG PET is the most common radiotracer used). Because MR excels at quantitative water and fat separation, PET images can be constrained such that activity is only present in the non-fatty tissue with a spatial resolution that matches the acquired MR images. We propose to develop such techniques for breast imaging, where the complex distribution of fatty and non-fatty tissue would enable PET imaging to benefit greatly from such a constraint.Methods
Using this biological phenomenon as a model, PET images can be constrained to an MR image with high spatial resolution that provides details on the distribution of fatty and non-fatty tissues. This can be readily approximated as a convolution model as described below:
$$ P = (P_{true} \cdot I_{wf} + b \cdot I_{ff}) \ast h $$
where P is the partial volume corrected PET image, Ptrue is the estimate of the true PET activity, Iwf is the fraction of tissue from the water or non-fatty compartment, b is a constant reflecting the background activity in the adipose tissue, Iff is the fraction of tissue from the fatty compartment, and h is the system blurring function of the PET system (modeled as a Gaussian blurring kernel). This is depicted graphically in Figure 1.
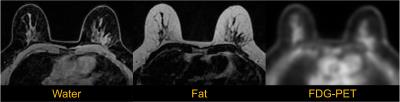
To evaluate this approach, images of the breast were obtained in an integrated scanner (Signa PET/MR, GE Healthcare, Waukesha, WI). Simultaneous PET and MR imaging were obtained in a patient volunteer (under IRB approval) and images were processed offline using MATLAB. The PET imaging protocol included 30 minutes of PET data. Reconstruction parameters were as follows: VPFX-S, 28 subsets, 3 iterations, 6mm post filter. In addition to conventional clinical contrast-enhanced breast MR acquisitions, an IDEAL-IQ scan with resolution 1.33x1.33x3mm was obtained to implement the methods proposed above. Example PET and IDEAL water and fat images are shown in Figure 2.
Results and Discussion
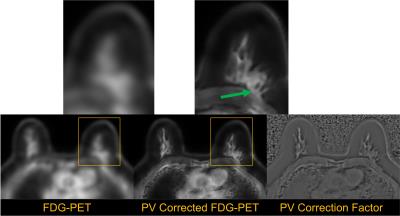
Figure 3 shows the result of the proposed water and fat-based partial volume correction. The resolution of the PET image is demonstrably increased, such that PET activity is confined within the fibroglandular tissue, and not white adipose tissue. Note that this method is similar in concept to previously reported approaches for PET neuroimaging (e.g., [1]). However, such methods have not to our knowledge been applied using water and fat segmentation. Other recent approaches in PET/MR [2] have utilized fat as a constraint directly in PET reconstruction. Regardless of whether the method is integrated into image reconstruction or utilized as a post-processing technique on reconstructed images, a significant value of the simultaneity of PET and MR imaging is the inherent and natural coregistration of the two modalities, which greatly facilitates the use of MR-based partial volume correction.Conclusion
As demonstrated herein, the use of deconvolution-based methods is technically feasible and merits future study. Approaches using water and fat-based segmentation are expected to be particularly value in breast imaging, where many fat and water interfaces define the anatomy. Furthermore, the use of methods to enhance the spatial resolution of PET are important, as the resolution of whole body PET scanners can limit detectability in breast PET imaging [3]. In future work, we expect to evaluate the quantitative accuracy of this approach in a cohort of patients presenting with known breast cancer lesions.Acknowledgements
No acknowledgement found.References
1. Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, Lin MP, Price JC. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 1999 Dec 1;40(12):2053–65. PMID: 10616886
2. Prevrhal S, Heinzer S, Wülker C, Renisch S, Ratib O, Börnert P. Fat-constrained 18F-FDG PET reconstruction in hybrid PET/MR imaging. J Nucl Med. 2014 Oct;55(10):1643–9. PMID: 25168626
3. Yamamoto Y, Ozawa Y, Kubouchi K, Nakamura S, Nakajima Y, Inoue T. Comparative analysis of imaging sensitivity of positron emission mammography and whole-body PET in relation to tumor size. Clin Nucl Med. 2015 Jan;40(1):21–5. PMID: 25423346
Figures