1323
The Effect of RF Exposure Duration in RF Pulse Design Using Temperature Constraints1Department of Radiology, Center for Advanced Imaging Innovation and Research (CAI2R) and Bernard and Irene Schwartz Center for Biomedical Imaging, New York University School of Medicine, New York, NY, United States, 2The Sackler Institute of Graduate Biomedical Sciences, New York University School of Medicine, New York, NY, United States
Synopsis
There is an increasing interest in using temperature to ensure safety in MRI. We designed parallel transmission RF pulses using either SAR or temperature constraints and compared to each other and unconstrained RF pulse design in terms of excitation fidelity and safety for four different RF exposure durations (from 6 mins to 24 mins). We found that the benefit of using temperature correlation matrices on RF pulse design diminishes as RF exposure duration increases. However, safety of the subject is always guaranteed (the maximum temperature was equal to 39°C). This trend was observed in both head and hip regions, where the perfusion rates are very different.
Purpose
RF safety of subjects is generally ensured during parallel transmission (pTx) RF pulse design using SAR1. Recently, the use of temperature constraints has been proposed during pTx RF pulse design to ensure patient safety2–4. Various pTx RF pulse strategies have been implemented using electromagnetic(EM) field simulations of head models: where the moderating effects of perfusion are expected to enable superior RF pulse designs using temperature as a constraint compared to SAR. In the present study, we analyzed the effect of RF exposure duration (from 6 to 24 minutes ) on RF pulse design performance for regions with different perfusion characteristics: the head (with brain having relatively high perfusion rates), and the hip (containing only tissues with relatively low perfusion rates).Methods
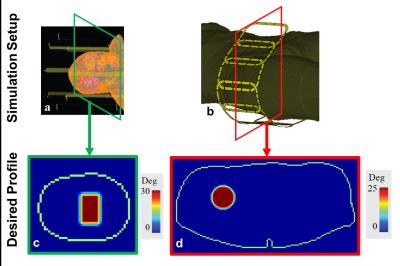
EM field simulations of 8 element 7T transmit hip (Fig.1a) and head arrays (Fig.1b) using the HUGO head model (resolution=5mm3) and the Ella body model5 (resolution=1mm3) were performed, respectively. Individual coil field distributions were used for RF pulse design and temperature simulations. pTx RF pulses were designed using the small-tip-approximation6 and the following convex optimization with virtual observation points(VOPs)7: $$\begin{matrix}&\widehat v_{full}=\begin{matrix}argmin\\v_{full}\end{matrix}\left\|A_{full}v_{full}-m_{des}\right\|^2_2\\\text{subject to}&T_{0,j}+v^{*}_{full}Z^{j}_{full}v_{full}\leq39°C\text{ }\forall j\end{matrix}$$ where $$$m_{des}$$$ is the desired magnetization profile, and $$$Z^j_{full}$$$ are the block diagonal matrices that contain temperature VOPs to be used in conjunction with $$$v_{full}$$$. $$$T_{0,j}$$$ is the maximum initial temperature located within the cluster of jth temperature VOP. RF pulses designed with temperature constraints (“temperature constrained”, with max 39°C) were compared to RF pulses designed with 10g averaged SAR constraints (“10gSAR constrained”, with max 10W/kg) as well as RF pulses designed without any constraints (“unconstrained”). Details of obtaining the temperature correlation, $$$Z_{full}$$$, and system, $$$A_{full}$$$, matrices are explained in Ref.2. A constant rate spiral-in excitation k-space trajectory was used in a gradient-echo-based sequence with TR=9ms with the following parameters: duration=2.2ms(hip)/3.1ms(head), sampling interval=10μs, maximum gradient slew rate=150mT/m/s and gradient amplitude=40mT/m. Desired excitation profiles are shown in Fig1.c-d.Results
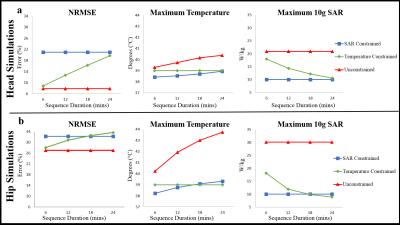
Figure.2 shows results from RF pulses designed for different RF exposure durations in head and hip regions. In both regions, RF pulses designed without safety constraints resulted in constant NRMSE and the maximum SAR for all exposure durations, and the maximum temperature increasing with exposure duration reaching 40.4°C and 43.7°C at the end of 24mins of imaging for head and hip regions, respectively. In 10gSAR constrained design, the maximum temperature increased from 38.4°C to 38.9°C and 38.2°C to 39.3°C for head and hip regions, respectively, while NRMSE and the maximum SAR were constant as the exposure duration is increased.
Contrary to the trends observed for unconstrained and 10gSAR constrained pulses, in the temperature constrained design for the hip region, the NRMSE increased (from 28.0% to 33.6%) and the maximum SAR decreased (from 18.2W/kg to 8.9W/kg) as RF exposure duration is increased. In the head, the NRMSE increased (from 8.8% to 19.7%) and the maximum SAR decreased (from 18.0W/kg to 10.6W/kg) as the RF exposure duration is increased. In both regions, the maximum temperature remained constant at 39°C as RF exposure duration changed. To meet strict temperature safety requirements, some compromise in NRMSE was required during temperature constrained RF pulse design. For sequence durations up to 18mins and above 24mins, lower NRMSE was obtained using strict temperature constraints compared to SAR constraints in the hip and head, respectively. For both higher benefits of using temperature constraints are obtained for shorter sequence durations.
Discussion and Conclusions
We demonstrate that using temperature correlation matrices for pTx RF pulses constraining maximum absolute temperature is beneficial in both regions. Our results agree with previous investigations obtained in the head using constraints on temperature increase2,4, indicating that better excitation profiles can be achieved with temperature constraints than with SAR constraints. Regardless of the perfusion characteristics differences, our results indicate using temperature correlation matrices enable lower excitation errors especially for shorter RF exposures and still ensure patient safety. As the RF exposure duration is increased, the benefit of using temperature correlation matrices diminishes for both regions. However, safe operation is always guaranteed by using temperature correlation matrices with the expense of increased excitation error, while using strict SAR constraints may result in exposure conditions where the maximum local temperature exceeds 39°C. It is also important to note that continuous exposures to a single SAR distribution and level (single sequence) lasting up to 24mins is increasingly rare in practice. Rather, shorter sequences with different SAR levels are applied in series, often with breaks in between them during which temperature will progress towards baseline. Thus, we believe that the results for exposure periods shorter than 24mins are more relevant in practice.Acknowledgements
The Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at New York University School of Medicine is supported by NIH/NIBIB grant number P41 EB017183.References
1. International Electrochemical Commission. Medical electrical equipment: part 2-33. Particular requirements for the safety of magnetic resonance equipment for medical diagnosis. IEC 60601-2-33 ed 3.0. (Geneva: IEC) Consolidated with amendments (2010).
2. Deniz, C. M., Carluccio, G., Sodickson, D. K. & Collins, C. M. Non-Iterative Parallel Transmission RF Pulse Design with Strict Temperature Constraints. in In Proceedings of the 24th Scientific Meeting, ISMRM 549 (2015).
3. Boulant, N., Massire, A., Amadon, A. & Vignaud, A. Radiofrequency pulse design in parallel transmission under strict temperature constraints. Magn. Reson. Med. 72, 679–688 (2014).
4. Boulant, N. et al. Direct control of the temperature rise in parallel transmission by means of temperature virtual observation points: Simulations at 10.5 tesla. Magn. Reson. Med. 75, 249–256 (2016).
5. Christ, A. et al. The Virtual Family--development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys. Med. Biol. 55, N23-38 (2010).
6. Grissom, W. et al. Spatial domain method for the design of RF pulses in multicoil parallel excitation. Magn. Reson. Med. 56, 620–629 (2006).
7. Eichfelder, G. & Gebhardt, M. Local specific absorption rate control for parallel transmission by virtual observation points. Magn. Reson. Med. 66, 1468–76 (2011).
Figures