1320
2D Outer Volume Suppression with T2-Preparation and Fat Saturation for Coronary Angiography1Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
A sequence for combined 2D outer volume suppression (OVS) with T2-preparation and fat saturation is proposed. This sequence provides a uniform passband robust to inhomogeneities, significantly lower SAR than existing methods, and flexibility to be used as a standalone OVS sequence. Numerical simulation and phantom results verify the sequence in theory and in vivo results corroborate the performance in practice.
Purpose
Outer volume suppression (OVS) with T2-preparation for coronary magnetic resonance angiography reduces FOV and suppresses aliasing and motion artifacts to improve image quality and reduce scan time1,2. This work presents a new sequence for combined 2D OVS, T2-preparation, and fat saturation. Additionally, this sequence has the flexibility to be used as a standalone OVS sequence with minimal T2-preparation.Methods
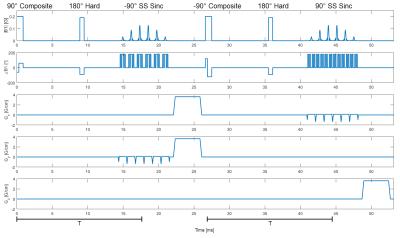
The sequence (Figure 1) begins with a 90° tip-down composite pulse3. A hard 180° refocuses the magnetizations orthogonally at T/2 for CPMG conditions4. At T, a -90° spectral-spatial sinc tips up the water magnetization5. The remaining transverse magnetizations are crushed by gradients on X and Y. The second half of the sequence negates in amplitude the tip-down and tip-up 90° pulses while the 180° pulse remains the same. Remaining transverse magnetizations are crushed by a Z gradient. There is a total T2 decay time of Tprep=2*T. Crushing while the desired magnetization is longitudinal rather than transverse is crucial for minimizing flow-related phase effects. The crushers are on orthogonal axes to prevent stimulated echoes.
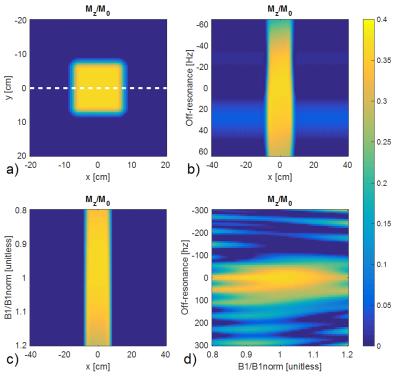
Bloch simulations of the sequence are shown in Figure 2 with T1/T2=1130/35ms to represent muscle at 1.5T6. Figure 2a shows the simulated OVS profile. Figures 2b-d indicate that the sequence is robust against B0 and B1 variations.
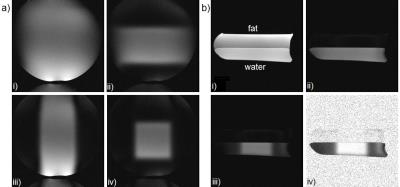
OVS in X and Y is achieved by the respective spatial selectivities of the two tip-up pulses. Figure 3 shows the profile when various combinations of gradients are played. Fat saturation is achieved by tipping down fat and then spectrally ignoring it with the tip-up pulse so that its magnetization remains transverse and is crushed.
The off-resonance and B1 robustness of the sequence stems from two factors. One factor is its symmetry such that B1 inhomogeneity will be compensated when the pulse is negated and repeated. Another factor comes from the composite tip-down pulse which already compensates for B1 variance by design and also has sufficient bandwidth to be robust to off-resonance and excite water and fat simultaneously.
To test the performance of this preparation sequence, experiments were conducted on a 1.5T scanner. Phantom and head scans were acquired with 2D GRE, FOV=28cm2, and an 8-channel head coil. Phantom scans use TE/TR=2/200ms and Tprep=35ms. Head scans use varying TE/TR for desired contrast, Tprep=6ms, and a modified sequence where spatial sincs replace the spectral-spatial sincs to minimize Tprep.
In vivo cardiac results were collected with a 3D cones trajectory7 with alternating-TR-SSFP, TE=0.5ms, TR1/TR2=4.484/1.196ms, FOV=28x28x14cm3, flip angle=70°, 1.2mm isotropic resolution, 2D OVS ROI=14.5x11.4cm2, and Tprep=35ms. The images are translationally motion-corrected based on 3D image-based navigators8.
Results
Simulation results for OVS and fat suppression are corroborated by the phantom results in Figure 3. Figure 4 shows in vivo head scans with various contrasts, demonstrating the sequence as a standalone OVS technique.
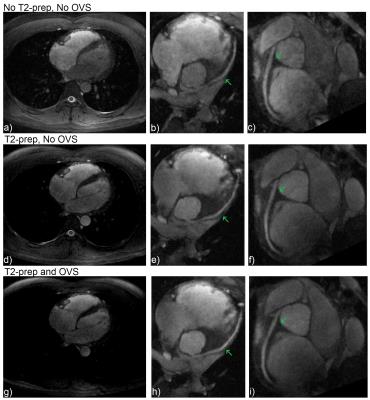
Figure 5 shows in vivo cardiac results. Figures 5d-i show enhanced blood-myocardium contrast from T2-preparation. Figure 5g clearly demonstrates suppression in the stopband chest muscle from OVS. Dividing the image of Figure 5g by Figure 5d, the chest muscle has been suppressed by a factor of 3.2.
For SNR calculations, we consider an ROI in the left ventricle. For CNR calculations, we compare the signal from the left ventricle with the signal from an ROI in the myocardium of the interventricular septum. Figure 5a (without preparation) has SNR=7.47, CNR=1.65. Figure 5d (T2-preparation, no OVS) has SNR=6.77, CNR=3.21. Figure 5g (T2-preparation, OVS) has SNR=7.42, CNR=3.46.
Discussion
These results demonstrate the ability of the sequence to provide 2D OVS, T2-preparation, and fat saturation. Initial cardiac results show improved CNR versus no T2-preparation and improved SNR versus no OVS, likely from suppressing aliasing and motion artifacts from surrounding material. Additional scans will be required to generate a rigorous statistical comparison between the methods.
An advantage of this sequence is its uniform, high TBW
passband. Alternative T2-preparation OVS sequences use 2D-spiral pulses which
result in sensitivity to inhomogeneities that distort the 2D passband profile9.
2D-spiral pulses also cannot achieve a high TBW in the allotted excitation
time. These apodization effects can negatively impact intensity-based motion
correction algorithms10. A more uniform passband also leads to lower
passband variance and thus better SNR. Additionally, alternative T2-preparation
OVS sequences involve adiabatic double refocusing excitations that result in
high SAR1,2. Compared to [1], the proposed sequence has 80% less
SAR.
Furthermore, the sequence is extensible such that the tip-up pulse can be a 2D pulse and custom pulses could be substituted to achieve different profiles and effects. Finally, this sequence has demonstrated its standalone OVS performance and further work will study additional applications.
Acknowledgements
This research is supported by NIH R01 HL127039, GE Healthcare, Joseph W. and Hon Mai Goodman Stanford Graduate Fellowship, and the National Science Foundation Graduate Research Fellowship under Grant No. DGE-114747.References
1. Luo J, et al. Combined Outer Volume Suppression and T2 Preparation Sequence for Coronary Angiography. Magn Reson Med. 2015;74(6):1632-1639.
2. Coristine AJ, van Heeswijk RB, Stuber M. Combined T2-preparation and two-dimensional pencil-beam inner volume selection. Magn Reson Med. 2015;74(2):529-536.
3. Levitt MH. Symmetrical composite pulse sequences for NMR population inversion. I. Compensation of radiofrequency field inhomogeneity. J Magn Reson. 1969;48(2):234-264.
4. Meiboom S, Gill D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev Sci Instrum. 1958:29(8):688-691.
5. Meyer C, Pauly J, Macovski A, Nishimura D, Simultaneous Spatial and Spectral Selective Excitation. Magn Reson Med. 1990;15(2):287-304.
6. Han E, et al. In-Vivo T1 and T2 Measurements of Muskuloskeletal Tissue at 3T and 1.5T. Proc. ISMRM 11. 2003:450.
7. Wu HH, et al. Free-breathing multiphase whole-heart coronary MR angiography using image-based navigators and three-dimensional cones imaging. Magn Reson Med. 2013;69(4):1083-1093.
8. Luo J, et al. Nonrigid motion correction with 3D image-based navigators for coronary MR angiography. Magn Reson Med. 2016.
9. Pauly J, Nishimura DG, Macovski A. A k-space analysis of small-tip-angle excitation. J Magn Reson. 1989;81(1):43-56.
10. Wu W, Koopmans PJ, Frost R, Miller KL. Reducing slab boundary artifacts in three-dimensional multislab diffusion MRI using nonlinear inversion for slab profile encoding (NPEN). Magn Reson Med. 2016;76(4):1183-1195.
Figures