1315
Application of retrospective motion correction to magnetic resonance fingerprinting1IMAGO7 Research Institute, Pisa, Italy, 2IMAGO7 Research Institute, 3IRCCS Stella Maris, Pisa Italy, 4Università di Pisa, Italy
Synopsis
The image quality of MRI exams is often poor in patients that cannot lay still or are unable to follow instructions. MR Fingerprinting is a novel technique with a greatly reduced sensitivity to patient motion and a short scan time. However, its images can still have artifacts when the degree of head movement is substantial. Here, we evaluate a novel motion correction scheme for MRF. Five subjects were instructed to intentionally move their head. In post-processing an iterative motion-correction algorithm was used on the anti-aliased image frames. When using our algorithm, we observed clearer images in all subjects, indicating that our technique can further increase the motion robustness of MRF.
PURPOSE
Movement of patients during MR exams is a frequent cause of image degradation, thus requiring repeated sequences or exams and increasing medical cost (1). Due to these limitations, young children have to be sedated during exams in order to obtain diagnostic images. Difficulties are not just limited to paediatric populations, but also affect many challenging adult populations. Recently, a new approach called MR fingerprinting (MRF) has been demonstrated for a fast quantitative assessment using MRI. This method has shown great promise as data can be acquired in a short time and are relatively insensitive to motion. In challenging populations, a standard anatomical MRI exam could potentially be replaced by a single MRF scan, as many different anatomical contrasts can be synthetized a-posteriori from MRF data (see figure 1). However, despite the relative motion insensitivity of MRF, maps obtained with severely corrupted frames still have artifacts. Here, we demonstrate an image-based motion-correction strategy following anti-aliasing of the MRF time series.METHODS
Acquisitions
Five healthy subjects underwent a 2D axial SSFP MRF acquisition [2] by using a 1.5T HDxt system (GE Healthcare, Milwaukee USA). We acquired interleaved 5mm slices with no gap, with 30cm FOV and 256x356 matrix. Variable density spirals [2] were incremented by the golden angle between frames [3]. Subjects were instructed to keep moving by rotating their heads by at least 30 degrees around the inferior-superior axis during scanning. The raw time-series consisted of 754 frames (imaging parameters shown in figure 2). Prior to motion correction, k-space view sharing was applied as described in [4].
Motion correction
To perform motion correction, an iterative approach was adopted. In each step, a filtered time-series (named F from here on) was generated by applying a 21-frame temporal moving average window to the raw data. The reference frame was set to frame 11 in F (the average of frames 1—21 in the raw data), this frame has high soft tissue contrast and given the short TI has minimal fat contamination. By using FSL’s FLIRT tool [5,6], the transformation matrix that best aligned the nth frame of F to the reference was applied to the nth frame of the raw data. The time-series obtained after this step was then used in a second iteration of the same algorithm. In this study, we only used two iterations of the algorithm.
Evaluation of images
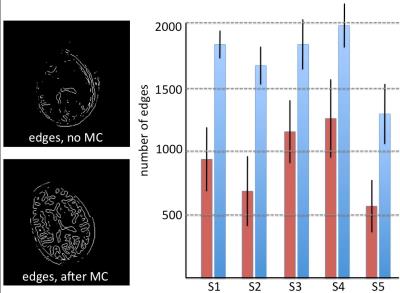
To evaluate the improvement obtained after motion-correction, the MRF T1 and T2 maps of both the raw data and after motion correction were reconstructed and visually inspected by a neuroradiologist. Since motion in the 754-frame time-series causes loss of anatomical detail and blurring, the amount of edges within the MRF T1 maps inside the brain was quantitatively evaluated by using a Laplacian-of-Gaussian (LoG) edge filter of size 13x13 and a standard deviation of 2.
RESULTS
Acquisition took 9 seconds per slice, for a total of less than 4 minutes to cover the whole brain. Figure 3 shows four example frames from the raw un-aliased 754-frame time-course of the central slice for one subject. The MRF T1 and T2 maps reconstructed from the raw and motion-corrected timeseries are shown in Figure 4. After motion correction, an improvement and increase in anatomical detail revealed by the LoG egde filter were observed. Figure 5 demonstrates the increment in detected edges at tissue interfaces in MRF T1 maps in each subject. On average, the number of edges detected inside the brain after motion correction was 1.98 times that of the edges detected on maps obtained without motion correction.DISCUSSION
We assessed retrospective motion correction of MRF frames. Our approach used existing software tools in order to achieve motion-corrected frames and in turn clearer parameter maps. We used averaged un-aliased timeframes to estimate motion parameters, that were then applied to individual image frames.
Although we showed a quantifiable improvement in image quality, some image artifacts remained, especially in the form of blurring, which may be due to intra-frame motion. It has to be emphasized that in this demonstrative experiment, to maximise the corruption of the images, the motion paradigm adopted by the volunteers represented an extreme case. Therefore, the residual motion artifacts may not be observed in future clinical applications. In addition, the algorithm studied here could be extended to include more iterations and regularization steps.
CONCLUSION
We demonstrated motion correction of MRF frames, leading to clearer frames and parameter maps. Our approach can further increase motion insensitivity of MR Fingerprinting for use in challenging patient populations.Acknowledgements
No acknowledgement found.References
1) Andre JB, Bresnahan BW, Mossa-Basha M, Hoff MN, Smith CP, Anzai Y, Cohen WA. Towards Quantifying the Prevalence, Severity, and Cost Associated With Patient Motion During Clinical MR Examinations. J Am College Radiol. 2015 Jul;13(7):689-695
2) Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med 2014;74:1621–1631. doi:10.1002/mrm.25559.
3) Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging 2007;26:68–76. doi:10.1109/TMI.2006.885337.
4) Buonincontri G, Sawiak SJ. MR fingerprinting with simultaneous B1 estimation. Magn Reson Med 2015. doi:10.1002/mrm.26009.
5) M. Jenkinson and S.M. Smith. A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2):143-156, 2001.
6) M. Jenkinson, P.R. Bannister, J.M. Brady, and S.M. Smith. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2):825-841, 2002.
Figures
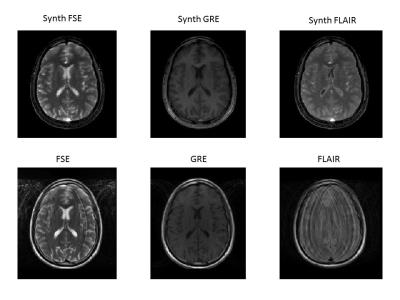
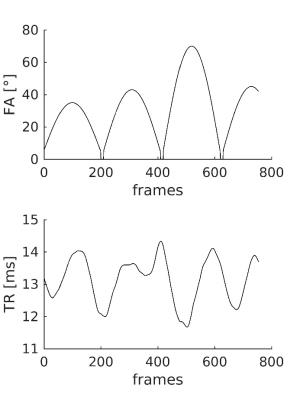
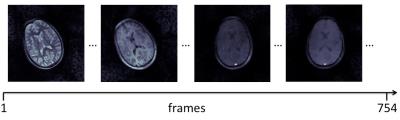
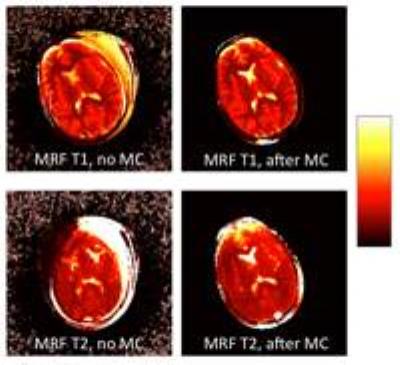
Figure 4. MRF T1 and T2 maps before and after motion correction on the same slice shown in Figure 3. T1 values range between 0 and 3s, T2 values range between 0 and 300 ms.