1312
Repeatability and motion-invariance of Zero Echo Time bone maps1GE Healthcare, Cambridge, United Kingdom, 2GE Global Research, Germany, 3GE Healthcare, United States
Synopsis
The goal of the present study was to evaluate the repeatability of a ZTE-based bone imaging method with respect to clinically realistic variations of the ideal acquisition conditions.
Purpose
A necessary step to obtain quantitative Positron Emission Tomography (PET) images is correcting for the attenuation introduced by the patient. In PET/CT systems, this can be achieved using the information provided by the CT. Combined PET/MR systems, on the other hand, rely on the segmentation of magnetic resonance (MR) data into a set of known tissue classes.
A known problem of this approach is the difficulty of acquiring reliable bone information with MR. Conventional sequences are unable to detect the free water in the osteons or bound to the organic matrix1,2, due both to the low proton density (~20% of water) and short signal lifetimes (T2 ~ 390 μs at 3T).
MR sequences capable of imaging tissues with short T2 relaxation times have been proposed as a means to account for bone attenuation in PET/MR systems3,4. In a recently published paper5, Wiesinger et al. postulate the use of proton-density-weighted Zero Echo Time (ZTE) acquisitions to create continuous density estimates of bone tissue for head attenuation correction. The goal of the present study was to evaluate the repeatability of such a method with respect to clinically realistic variations of the ideal acquisition conditions, such as nonstandard positioning of the patient head.
Methods
A research version of a 3D-radial ZTE sequence was installed on a GE SIGNA PET/MR scanner. The acquisition protocol was kept constant for all subsequent measurements: FOV 26.4x26.4x27.8cm, resolution 2.4x2.4x2.4mm3, acquisition time ~45seconds. A bandwidth of ±62.5 kHz was used to capture the fast-decaying bone signal. The FA was set to 0.8deg to minimize T1 saturation. In consequence, four averages were required.
The post-processing consisted of an inverse linear mapping of bias-corrected ZTE intensities. A head mask was used to eliminate non-patient voxels, while a second mask prevented false positive bone voxels in areas affected by partial volume (skin and sinuses). Subsequent steps, not considered for this study, would be resampling to PET resolution, mapping to 511 keV attenuation coefficients and incorporation of hardware templates.
Using this framework, multiple series of acquisitions were performed on healthy volunteer subjects, designed to test the repeatability of the method on increasing degrees of (clinically relevant) setup variation: Static subject acquired at different time points; Static subject acquired with varying axial centering of the ZTE field-of-view; Moving subject, head tilt; Moving subject, head rotation. The resulting bone maps were processed using a GE Advantage Workstation (for visualization and rigid registration) and custom Matlab code (for quantitative comparison).
Results
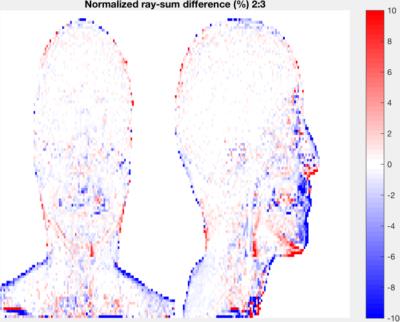
In static acquisitions, subtracted volumes were generated to identify differences between bone maps. Projection subtractions were also examined, being of particular relevance due to the projective nature of PET reconstruction. Figures 1&2 illustrate some of the results obtained. Bone estimation was found to be very consistent between acquisitions, with errors well below those introduced by involuntary patient motion during the course of a typical examination.
In the case of varying axial centering, results were similar as long as the field-of-view remained on the subject’s head. Shifting the acquisition towards the thorax was found to cause artifacts near the edges of the coil –outside the scope of this study–, and occasionally an erroneous localization of the sinusal region.
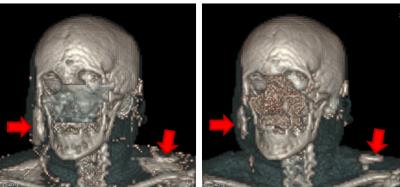
In rotated head measurements, bone maps remained consistent between acquisitions. False positive bone structures were found to appear when the subject overlapped regions of very high, localized sensitivity within the radiofrequency coil. Figure 3 illustrates this effect for a subject positioned with a head yaw.
Discussion
The results suggest that the existing bone imaging method is repeatable and suitable for clinical use in longitudinal studies. However, when considering non-ideal –but clinically realistic– acquisition conditions two issues were found in this study that required corrective measures:
Firstly, the identification of the sinusal region was found to fail when the field-of-view extends to the thorax. Secondly, bias overcorrection around areas of very localized high coil sensitivity was found to cause false-positive bone structures, typically on the cheeks and jaw of patients positioned with their heads rotated towards the side of the coil.
While the former issue can be readily prevented by taking into account information available in the ZTE metadata, only partial mitigation has been found for the latter, by improving the bias correction approach. Work is underway to address these false positives before taking the algorithm forward to product.
Conclusion
The bone imaging method evaluated in this study yielded repeatable results under standard acquisition conditions. Nonstandard patient positioning or large FOV shifts where found to cause changes in the bone maps. Mitigation strategies were implemented to account for these cases.Acknowledgements
No acknowledgement found.References
1. Du, J., and Bydder, G.M.: ‘Qualitative and quantitative ultrashort-TE MRI of cortical bone’, NMR Biomed, 2013, 26, (5), pp. 489-506 2. Bae, W.C., Chen, P.C., Chung, C.B., Masuda, K., D'Lima, D., and Du, J.: ‘Quantitative ultrashort echo time (UTE) MRI of human cortical bone: correlation with porosity and biomechanical properties’, J Bone & Mineral Res, 2012, 27, (4), pp. 848-857 3. Keereman, V., Fierens, Y., Broux, T., De Deene, Y., Lonneux, M., and Vandenberghe, S.: ‘MRI-based attenuation correction for PET/MRI using ultrashort echo time sequences’, J Nucl Med, 2010, 51, (5), pp. 812-818 4. Catana, C., van der Kouwe, A., Benner, T., Michel, C.J., Hamm, M., Fenchel, M., Fischl, B., Rosen, B., Schmand, M., and Sorensen, A.G.: ‘Toward implementing an MRI-based PET attenuation-correction method for neurologic studies on the MR-PET brain prototype’, J Nucl Med, 2010, 51, (9), pp. 1431-1438 5. Wiesinger F, Sacolick LI, Menini A, Kaushik SS, Ahn S, Veit-Haibach P, Delso G, Shanbhag DD.: ‘Zero TE MR bone imaging in the head’, Magn Reson Med, 2016, 75, (1), pp. 107-14.Figures