1304
A Comparison of Prospective Motion Correction with 19F NMR Field Probes and an Optical Camera1High Field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Institute for Microelectronics, University of Ulm, Ulm, Germany, 3Danish Research Centre for Magnetic Resonance, Copenhagen University Hospital Hvidovre, Copenhagen, Denmark, 4Department of Biomedical Magnetic Resonance, University Clinic Tuebingen, Tuebingen, Germany
Synopsis
This work shows a comparison of prospective motion correction using NMR field probes and an optical tracking system in a phantom with induced motion as well as in an initial in vivo experiment. Tracking results for both systems were recorded concurrently to compare the motion estimates. The prospectively corrected images of a moving phantom and a moving human subject show a comparable correction ability for both systems. However, the lower precision of the field probe based system might prevent an application in highest-resolution imaging.
Introduction
Motion artefacts are a major problem for functional and anatomical MRI. Many different methods have been proposed to overcome this obstacle1. The state of the art in head motion correction is prospective motion correction using a tracking modality of choice while updating slice positions and orientations in real-time during the acquisition. Optical tracking devices have a lot of advantages such as high accuracy and being almost independent from imaging sequences. However, they require direct line of sight to an optical target and are still expensive. A lower cost alternative with no line of sight necessary are NMR field probes2, although in most cases they require either special imaging sequence types or changes to the sequence in order to track their location3.
This work compares the prospective motion correction capabilities of an optical MPT (Moiré-Phase-Tracking) camera-marker system4 and 19F NMR field probes in a moving phantom and in an initial in vivo experiment.
Methods
The field probe motion tracking setup consists of 4 NMR magnetometers using Hexafluorobenzene (C6F6) as an active sample. The setup is a custom built standalone system with dedicated hardware to communicate with the probes and the scanner5. A more detailed description of the setup can be found in 6.
The optical tracking system is commercially available (Kineticor Inc, HI, USA) and uses an MPT marker for motion tracking. Both systems use an external PC which sends the calculated motion parameters to the scanner via UDP. There, it is handled by a custom built library in order to update slice positions. The MR scanner used in this experiment was a 9.4 T human scanner (Siemens, Erlangen, Germany).
For the phantom measurements the MPT marker and four field probes were fixed to a structure phantom. Three images without motion and three with induced motion were acquired subsequently (GRE 128x128, resolution 1.6 mm ³, TR 100 ms, TE 7.7 ms). The first one without correction, then, two images with prospective correction enabled using field probes and optical tracking respectively.
For in vivo testing, all measurement steps were repeated in a healthy volunteer (GRE 144x144, resolution 1.6 mm ³, TR 100 ms, TE 7.7 ms). Therefore, the MPT marker was attached to a subject specific bite-bar in order to have line of sight to the camera despite a shielded coil. The field probes were attached to the nose bridge and the temples of the subject to minimize the effect of skin movements on the motion measurement.
Results
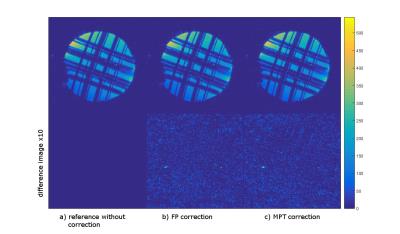
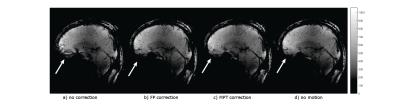
The difference images (Fig. 1) show that both correction methods introduce close to no artefacts into the image when the prospective correction is enabled but there is no induced motion.
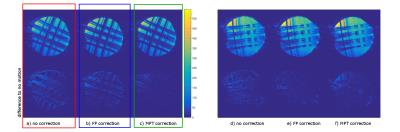
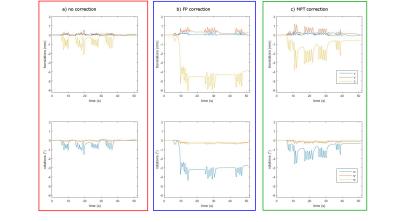
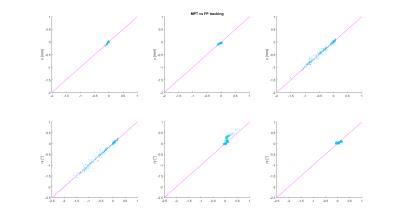
The measurements with induced motion (Fig. 2) show that prospective correction with both systems improves the image quality substantially. In the difference images however, residual motion artefacts are visible. The amount of residual artefacts varies between measurements but the result of the correction is comparable between the two systems. Since the motion was induced manually, the trajectories are not the same for all measurements (Fig. 3), but they show a similar pattern and range of motion. Fig. 4 shows a scatter plot comparing the measured motion parameters of the two systems against each other for one measurement. The average RMS deviation was 82 µm for translations and 0.04 degrees for rotations with a maximum deviation of the two systems of 444 µm for translations and 0.32 ° for rotations.
The in vivo measurements (Fig. 5) also show a reduction of motion artefacts with the field probe correction comparable to the MPT correction and close to the image without voluntary motion.
Discussion & Conclusion
NMR field probe based prospective motion correction is shown to improve image quality and comes close to the correction ability of the optical tracking system. However, since the precision in the current implementation is an order of magnitude less than the camera’s6, for highest-resolution images the camera might be the better choice. Furthermore, the sequences need to be changed to incorporate localization gradients (~4 ms) and due to calculation time the tracking frequency is at the moment limited to 14 Hz.
Field probe correction can be a cheaper alternative to optical tracking. It might also be used when a line of sight to the optical marker is not possible, a camera cannot be installed in the scanner bore due to space limitations (e.g. shim inserts, narrow head gradient systems) or when the camera’s field of view is too small for the expected motion since field probe tracking is in principle only limited by gradient dimensions.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (TH 1330/5-1 & SCHE 658/4-1).References
1. Maclaren, J. et al. Prospective motion correction in brain imaging: A review. MRM, (2013) 69: 621–636.
2. Barmet, C. et al. A transmit/receive system for magnetic field monitoring of in vivo MRI. MRM, (2009) 62(1): 269-276.
3. Haeberlin, M. et al. Real-time motion correction using gradient tones and head-mounted NMR field probes. MRM, (2015) 74: 647-660.
4. Maclaren J. et al. Measurement and Correction of Microscopic Head Motion during Magnetic Resonance Imaging of the Brain. PLoS ONE, (2012) 7(11): e48088.
5. Handwerker, J. et al., IEEE Biomedical Circuits and Systems Conference (BiOCAS), Rotterdam, The Netherlands, (2013) ID 5027.
6. Eschelbach, M. et al. A Comparison of 19F NMR Field Probes and an Optical Camera System for Motion Tracking. Proc. ISMRM 24, Singapore, (2016) 0340.
Figures