1295
Motion-corrected image reconstruction of abdominal DCE-MRI images1Department of Radiation Oncology, University of Michigan, Ann Arbor, MI, United States, 2Department of Radiology, University of Michigan, Ann Arbor, MI, United States, 3Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
Synopsis
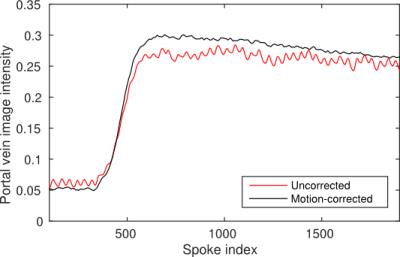
Respiratory motion of abdominal organs causes motion artifacts, blur and signal loss in DCE-MRI images which can confound liver perfusion quantification. To correct for respiratory motion a motion signal derived using rigid-body image registration was used to group acquired data from a golden-angle stack-of-stars sequence into motion states. These were then aligned using deformable image registration and the resulting deformation vector fields were used to deform complex projection images reconstructed from individual spokes. Deformed projections were finally combined using view-sharing into an image time series. The resulting portal-venous input function exhibited a higher signal enhancement and no breathing-induced intensity ripples.
Purpose
Liver DCE-MRI can be used to quantify portal-venous and arterial perfusion for assessing liver function and intrahepatic cancer progression during radiotherapy (1,2). However, respiratory motion of the liver and other abdominal organs causes motion artifacts, blur and signal loss in DCE images series. Deformable image registration can in part compensate for misalignments between frames but cannot remove blurring and artifacts in individual images. Motion-induced blur and signal loss distort contrast-agent uptake curves and therefore confounds perfusion estimation. In this work we present a technique that corrects motion in the DCE-image reconstruction and thereby improves quality of reconstructed images.Methods
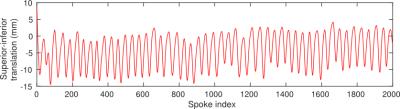
A 3D golden-angle stack-of-stars gradient-echo sequence (3,4) was used to scan a subject for 3 min during free breathing. After 30 seconds of scanning 20 ml of Gd-BOPTA was administered intravenously. A total of 2000 radial spokes were acquired and initially reconstructed using view sharing into a 2000-frame image time series with low spatial resolution but high temporal resolution. Region-limited rigid-body image registration was used to align the time-series images with respect to the liver. The translation of the liver in the superior-inferior direction, as indicated by the rigid-body registration transforms, was then used to sort radial spokes into 21 groups that were reconstructed into motion-state images ranging from inhale to exhale. The motion-state images were aligned to the exhale state using deformable image registration (5). The 21 deformation vector fields produced by the deformable registration were interpolated into 2000 vector fields using the previously derived superior-inferior position of the liver for each spoke. These vector fields were then used to deform complex projection images reconstructed from individual spokes. After deformation, projection images were combined using a view-sharing tornado filter in k-space (6). A view-sharing reconstruction of non-deformed projections was also performed for comparison.Result
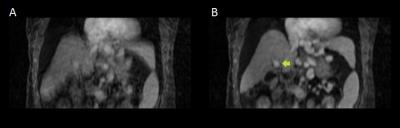
Registration of the time series with high temporal resolution resulted in a superior-inferior translation as shown in Fig. 1. View-sharing reconstruction of the deformed projection images produced an image time series without breathing motion or motion blur (Fig. 2). The portal-venous input function in the motion-corrected times series was smoother and had a higher signal enhancement as seen in Fig. 3.Conclusion
Motion-corrected reconstruction reduced motion blur in images and increased the amplitude of the portal-venous input function. These effects may improve the accuracy of portal-venous and arterial perfusion maps estimated from DCE-MRI images.Acknowledgements
This work is supported in part by NIH/NCI RO1 CA132834 and PO1 CA059827.References
1. Wang H, Farjam R, Feng M, Hussain H, Ten Haken RK, Lawrence TS, et al. Arterial Perfusion Imaging-Defined Subvolume of Intrahepatic Cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):167–74.
2. Cao Y, Wang H, Johnson TD, Pan C, Hussain H, Balter JM, et al. Prediction of liver function by using magnetic resonance-based portal venous perfusion imaging. Int J Radiat Oncol Biol Phys. 2013;85(1):258–63.
3. Block KT, Chandarana H, Milla S, Bruno M, Mulholland T, Fatterpekar G, et al. Towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. J Korean Soc Magn Reson Med. 2014;18(2):87–106.
4. Chandarana H, Block KT, Winfeld MJ, Lala S V., Mazori D, Giuffrida E, et al. Free-breathing contrast-enhanced T1-weighted gradient-echo imaging with radial k-space sampling for paediatric abdominopelvic MRI. Eur Radiol. 2014;24:320–6.
5. Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, et al. Fast free-form deformation using graphics processing units. Comput Meth Prog Bio. 2010;98(3):278–84.
6. Barger A V, Block WF, Toropov Y, Grist TM, Mistretta CA, Mra T. Time-Resolved Contrast-Enhanced Imaging With Isotropic Resolution and Broad Coverage Using an Undersampled 3D Projection Trajectory. Magn Reson Med. 2002;305:297–305.
Figures