1294
Synthetic MRI with Prospective Motion Correction1Radiology, Stanford University, Stanford, CA, United States, 2Global MR Applications & Workflow, GE Healthcare, Rochester, MN, United States, 3Global MR Applications & Workflow, GE Healthcare, Menlo Park, CA, United States
Synopsis
Quantification of tissue parameters such as T1, T2 and PD has gained significant interest due to potential applications in characterization of certain pathologies (Alzheimer’s, multiple sclerosis, etc.). One such technique, called “Synthetic MRI”, utilizes a fast spin echo (FSE) readout with inversion recovery in order to simultaneously get T1, T2 and PD maps using a single scan. In this abstract, we demonstrated that application of optical motion correction to simultaneous T1, T2 and PD quantification.
Introduction
Quantification of tissue parameters such as T1, T2 and PD has gained significant interest due to potential applications in characterization of certain pathologies (Alzheimer’s, multiple sclerosis, etc.). One such technique, called "Synthetic MR", utilizes a fast spin echo (FSE) readout with inversion recovery in order to simultaneously get T1, T2 and PD maps using a single scan [1]. Due to longer-than-usual scan time (~6 minutes) and inherent sensitivity of FSE techniques to even minuscule involuntary head motion, quantification accuracy can be increased by application of motion correction techniques. In this abstract, we demonstrated that application of optical motion correction to simultaneous T1, T2 and PD quantification.Methods
All experiments were performed on a 1.5T GE MR450w using the Magnetic Resonance Image Compilation (MAGiC®) platform. A multi-delay multi-echo FSE readout with inversion recovery was used to obtain T1, T2 and PD values [1]. Using these tissue parameters, synthetic T1-FLAIR, T2, T2 FLAIR and phase-sensitive IR (PSIR) images were generated. This acquisition technique does not have enough idle time to insert MR-based navigators such as PROMO [2] or vNavs [3]. Therefore, an external camera based motion tracking technique was used [4]. In this system, an optical pattern was attached on the volunteer’s forehead and tracked by a camera mounted on the head coil. 6-DOF motion was determined using real-time processing and the scanner geometry was updated immediately such that the scan plane “was locked” onto the volunteer’s forehead. The volunteer was asked to perform head rotation at discrete intervals. The scan was repeated four times, with the subject instructed to move/remain still and with the optical motion correction system turn off/on: 1) No motion, motion correction turned off, 2) No motion, motion correction turned on, 3) With deliberate head motion, motion correction turned off, 4) With deliberate head motion, motion correction turned on.Results
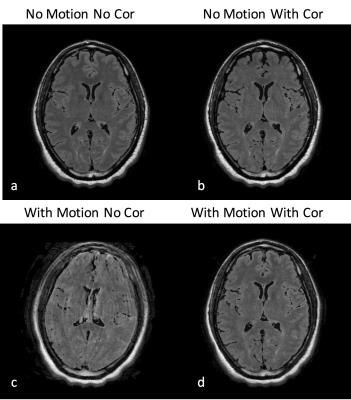
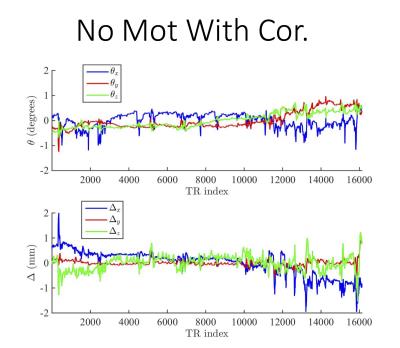
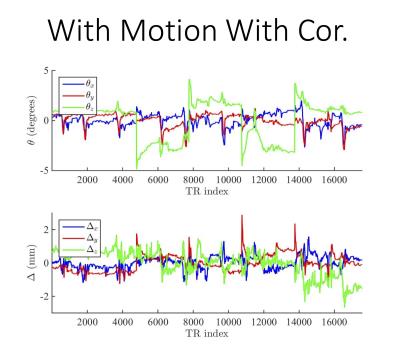
Figures 1 and 2 show the reconstructed synthetic PSIR and T2-FLAIR images, respectively. The four images in each figure correspond to the four scans as described above. Figures 3 and 4 show the detected motion for scans 2 and 4 described above.Discussion and Conclusions
In the presence of motion, application of motion correction improves the image quality from being non-diagnostic to diagnostic (Figs 1 and 2). Even when the subjects are cooperative and instructed to remain still, significant motion can occur, which can result in misregistration between multiple volumes used for calculation of tissue parameters (Fig 3). Despite the motion-corrected images remain diagnostic, slight residual artifacts remain possibly due to two reasons: 1) No motion updates were performed within the echo train (intra-TR motion was not corrected) and 2) Fast-motion can result in additional phase, which might violate the CPMG condition. These can potentially be addressed by updating the scanner geometry within the FSE readout or post-processing.Acknowledgements
NIH (2R01 EB00271108-A1, 5RO1 EB008706, 5R01 EB01165402-02), the Center of Advanced MR Technology at Stanford (P41 EB015891), Lucas Foundation, Oak Foundation, GE Healthcare.References
[1] Warntjes JBM, Dahlqvist O, Lundberg P. Novel method for rapid, simultaneous T1, T2*, and proton density quantification. Magnetic Resonance in Medicine 2007;57:528–37. doi:10.1002/mrm.21165.
[2] White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, et al. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magnetic Resonance in Medicine 2010;63:91–105. doi:10.1002/mrm.22176.
[3] Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic Resonance in Medicine 2011;68:389–99. doi:10.1002/mrm.23228.
[4] Aksoy M, Forman C, Straka M, Skare S, Holdsworth S, Hornegger J, et al. Real-time optical motion correction for diffusion tensor imaging. Magnetic Resonance in Medicine 2011;66:366–78. doi:10.1002/mrm.22787.
Figures