1291
Correction of Ghosting Due to Respiration-induced B0 Variation in Double Echo Steady State (DESS) Breast Imaging: Initial Validation1Radiology, Stanford University, Stanford, CA, United States
Synopsis
Double Echo Steady State (DESS) breast imaging for T2 and diffusion-weighted imaging in the breast is limited due to prominent ghosting artifacts. While actual respiratory motion of the breast tissue is minimal due to the prone position of the patient as well as immobilization paddles on the coil, respiratory-induced B0-variation is a source of ghosting. A method based on a per-TR off-resonance estimate and simulated phase data for correction of ghosting artifacts in DESS in the breast is described and validated in eight breast cancer patients. The new method effectively reduces ghosting artifact greatly improving image quality.
Purpose
To present and validate a method for correction of ghosting due to respiration-induced time-varying off-resonance in double-echo steady-state (DESS) breast imaging.Introduction
The role of MRI in breast imaging continues to evolve including interest in alternate contrasts [1]. An investigation of Double Echo Steady State (DESS) in the breast for T2 and diffusion-weighted contrast was promising [2] but limited by the presence of prominent ghosting artifacts.
Motion of the breast tissue during MRI is very restricted [3]. In DESS imaging, however, respiration-induced B0 variation results in ghosting. A previously developed method [4] showed promising but inconsistent reduction of ghosting in DESS in the breast. In this work we present a modified version of this correction method and validate it in 8 breast cancer patients.
Methods
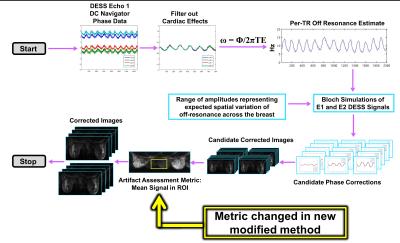
The original method acquires a center of k-space navigator before the first echo in DESS to estimate per-TR off-resonance variation. This estimation is incorporated into Bloch simulations to determine a per-echo, per-TR phase correction (Figure 1). To account for off-resonance spatial variation [3] multiple simulations are run with scaled versions of the off-resonance estimate, resulting in a set of candidate phase corrections.
The primary modification between the initial version of the method and the modified method, is the metric to assess level of artifact in the candidate corrected images. Originally, an image entropy metric [5] was utilized. In the modified method, artifact correction is assessed based on the mean signal within an ROI between the breasts. This metric determines the candidate phase correction providing the largest reduction in artifact for each coil-slice-echo combination. These images are combined for the final corrected set of images.
The modified method was tested in 8 breast cancer patients following IRB protocols. DESS acquisitions with the navigator were acquired on a GE Discovery MR750 3T (GE Healthcare, Waukesha, WI) with a 16-channel Sentinelle breast coil (Invivo, Gainesville, FL). FOV 36 to 40 cm, 256 x 256 matrix, 56 to 64 slices, 3 mm slice thickness, TR 17 ms, TE1 6 ms, TE2 28 ms, diffusion gradient 31.32 mT s/m and no parallel imaging.
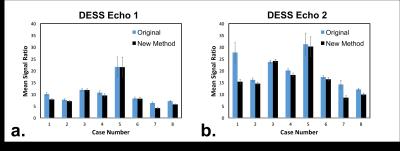
To assess the difference in artifact between the corrected images and original images, mean signal ratio was calculated between the regions of the image without and with breast tissue (Figure 2). This ratio was calculated for a set of 11 slices at the center of each dataset and then averaged to assess the consistency of the correction across multiple slices. Images with a lower mean signal ratio should have a lower level of artifact.
Results
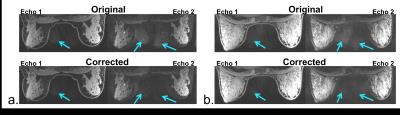
The mean signal ratio between regions of the image without and with breast tissue averaged across 11 central slices was reduced or remained equivalent in both echoes for all cases except for echo 2 in one case (Figure 3). The percent reduction ranged from 0.3% to 22% in echo 1 and from 3% to 44% in echo 2. The reduction in artifact, particularly in echo 2 where the artifact is more prominent, noticeably improved image quality (Figure 4).
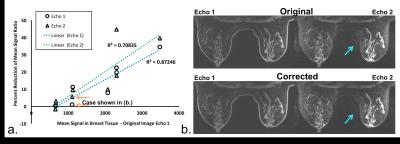
The smaller reduction in mean signal ratio in some cases does not indicate a failed correction but rather are cases in which initial artifact is low. The level of ghosting artifact can vary greatly from case to case based on the amount of fibroglandular tissue in each patient. The percent reduction in both echo 1 and echo 2 was correlated with the mean signal in the breasts in the original echo 1 image (Figure 5). This correlation indicates that significant reduction of artifact occurred in cases with prominent ghosting artifact while cases with little or no ghosting artifact were appropriately unaffected by the correction method.
Discussion
The original method showed promise because the different candidate phase corrections resulted in varying artifact correction [4], however, the candidate images with the lowest level of artifact were not being correctly identified. The use of the mean signal metric in the modified method more consistently chooses the candidate images with the lowest level of artifact.
Cardiac effects were present in the one case in which the correction method increased the level of artifact. Ghosting due to cardiac motion or respiration-induced B0 varies from case to case. Future studies will include the combination of this method with methods to correct for ghosting due to breast tissue motion.
Conclusion
The proposed method effectively corrects for ghosting due to respiration-induced B0 variation in both echo 1 and echo 2 DESS breast images in 7/8 cases analyzed and shows promise as a method to greatly improve image quality in DESS acquisitions in the breasts.Acknowledgements
Research support from GE Healthcare and NIH/NIBIB R01 EB009055 and NIH/NIAMS R01 AR0063643References
1. Kim JH, et al., Radiology 2016; DOI: 10.1148/radiol.2016160261. 2. Granlund KL, et al., MRI 2014; 32:330-41. 3. Bolan, et al., Mag Reson Med 2004; 52: 1239-45. 4. Moran CJ, et al., 24th Annual Meeting and Exhibition of the ISMRM, 2016, p. 4285. 5. Atkinson D, et al., Mag Reson Med 1999; 41:163-70.Figures