1287
How to correct block design task-induced coherent head motion; Evaluation of retrospective motion correction methods in HCP fMRI1Radiology, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Participants in block design finger tapping fMRI have a tendency to have paradigm coherent head motion. This finding appears to be very strong in HCP data, which utilizes spatially and temporally accelerated SMS sequence. We have introduced slice-oriented motion correction method (SLOMOCO1), and found SLOMOCO removed the head motion efficiently in HCP data, especially in the case that head motion pattern is synchronized to task paradigm. In this study, we compared the various motion correction methods in finger tapping fMRI HCP data.
Purpose
To correct block design task paradigm-coherent head motion in fMRI HCP data and to evaluate various retrospective head motion correction methods in HCP fMRIMethod
Multiple Sclerosis patients were scanned in 3T (N=5) and 7T scanner (N=4) using HCP protocols. During SMS EPI scanning, bilateral complex finger tapping was performed with 4 times of ON/OFF block design with 45s of duration.
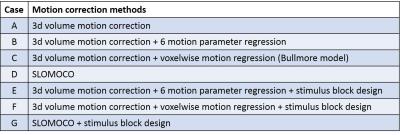
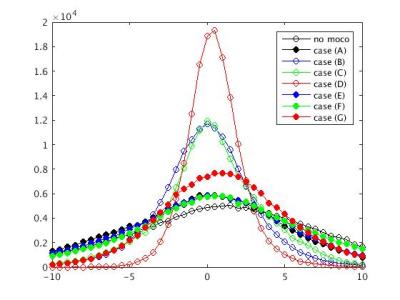
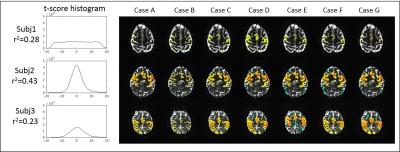
After removal of the first 10 volumes, 3d volume correction was conducted using 3dvolreg in AFNI2, and 6 rigid motion parameters of each volume were calculated. Six different motion correction techniques were applied, as outlined in Table1 (Fig1). Case (A) is 3dvolreg alone, Case (B) is regression of 6 motion parameters from each volume, Case (C) is regression of the voxel level displacement calculated from the 6 motion parameters3, Case (D) is the application of SLOMOCO1, which includes in-plane motion correction and out-of-plane regression. (E-F) are similar to B-D, except that the stimulus block design model is embedded such that only the motion that has a non-zero projection onto the stimulus paradigm (in the vector formalism) is regressed from the data. Thus, E-F, in principle, only regress motion parameters that have non-zero correlation with the paradigm, which are commonly used in fMRI analysis. Using 3dDeconvolve, a t-score activation map was generated, and whole brain t-score distribution is normalized and converted to a z-score because each post-processing pipeline will have different degrees of freedom (see Fig2). Additionally, the whole brain t-score histogram without any motion correction was presented to provide a visualization of the task activation and motion artifact. Finally, to estimate the coherence of motion to the task of each scan, the temporal correlation between the task paradigm timing and 6 motion parameters was calculated.Result
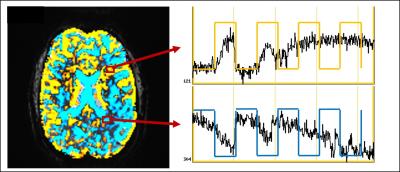
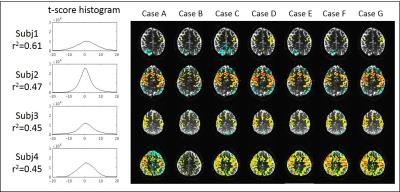
Figure 3 shows the representative motion corrupted activation map (p<10-4) before any retrospective motion correction from subject 1 at 3T. Subject 1 at 3T shows the highest correlation of the head motion and the task paradigm with r2 of 0.61. The time series signal in most brain areas are synchronized positively or negatively to the finger tapping paradigm, a pattern often seen with task coherent head motion. SLOMOCO locates the activation in the motor cortex with the highest z-score (shown in Fig 4) while other motion correction methods present the negative coherent pattern in the posterior brain, which is suspected as motion related artifact.
Figure 4 and 5 show the z-score activation maps (p<10-3) in 3T and 7T, with different head motion correction post processing. The effect of task coherent head motion is greatest when only volumetric realignment (e.g. 3dvolreg motion correction, or case A) is used. The incorporation of any motion parameter regression improves the statistical significance of brain activation.
Discussion
Despite the well-known impact on block design fMRI from motion, particularly for increased false positive rates4, 5, block design paradigm is still commonly used in fMRI. This is largely due to the much larger contrast-to-noise for activation from these study designs, compare to event-related designs6. However, it has been a challenge to correct the task coherent motion related to signal change in fMRI analysis. The conventional retrospective motion correction methods assume the head motion between volume acquisitions but not between slice acquisitions. SLOMOCO was introduced to deal with sub-volume head motion by allowing slices to move independently. This qualitative evaluation of different head motion correction methods supports the use of sub-volume motion correction such as SLOMOCO for task paradigm fMRI.
The degree of correlation between the motion and task paradigm indicates the coherence of the motion artifact to the task, and could be used as the threshold to exclude false positives. Also, the whole brain t-score distribution before motion correction could reflect the quality of the data with respect to motion, which is shown by two contrasting distributions: narrow distributions in subject 2 at 3T and 7T, possibly reflecting less motion, and broad distributions in subject 1 at 3T and 7T, possibly reflecting more motion.While motion parameter regression that includes a task paradigm in the model (e.g. cases E, F, G) improves the statistical significance of the activation in most of the presented cases, the case of the severe task coherent motion, e.g. subject1 at 3T, shows that a regression-based correction that includes the task paradigm prevents actual BOLD activation being detected by a later regression analysis.Conclusion
Motion parameter regression improves the statistical significance of true activation, and decreases false positive activation induced by motion artifact. SLOMOCO shows the potential to improve task coherent motion artifact.Acknowledgements
Authors gratefully acknowledge Dr. Essa Yacoub for CMRR C2P support (CMRR_MB_EPI_VD13D_R014, CMRR_MB_EPIVB17A-UHF_R014), and HCP community for the helpful discussion.References
1. Beall EB, Lowe MJ. SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 2014;101:21-34.
2. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-73.
3. Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SC, Sharma T, McGuire PK. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Human brain mapping. 1999;7(1):38-48.
4. Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Human brain mapping. 1999;7(2):106-14.
5. Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23(3):1046-58.
6. Liu TT. The development of event-related fMRI designs. Neuroimage. 2012;62(2):1157-62.
Figures