1283
Prospective Self-Gating For Cardio-Respiratory Synchronised Imaging in the Mouse.1Oxford Institute for Radiation Oncology, University of Oxford, Oxford, United Kingdom
Synopsis
Prospective cardio-respiratory self-gating is demonstrated in the mouse. A gradient echo scan operating at constant TR enabled acquisition of CINE data blocks or maintenance of the NMR steady state depending on the level of a gating control signal that was evaluated within each TR. A portion of the FID during each TR was submitted to a signal processor chain for streaming into a pseudo-continuous analogue cardio-respiratory signal trace and conversion to a series of logic control signals for gating.
Introduction
Retrospective gating, using the NMR signal itself to drive the reconstruction data selection, is widely used as a means of reducing the cardio-respiratory (CR) motions without the need for external signal detection.1-4 However, retrospective gating scans are long due to the need to acquire multiple repeats of an imaging volume for post hoc data sorting. Prospective self-gating has been used to improve the scan efficiency in clinical MRI as a means of improving efficiency.5 Here, we describe a simple apparatus that enables the use of the NMR signal itself to drive prospective CR self-gated imaging in the mouse using standard commercial gating controllers on a standard commercial preclinical MRI system.Methods.
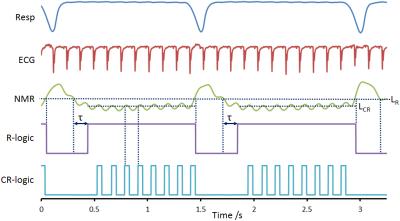
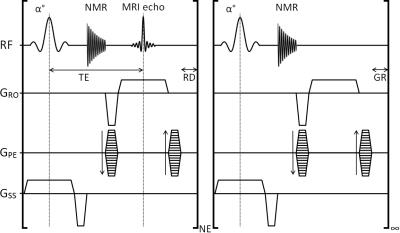
MRI was performed at 4.7 T (Varian VNMRS) with the NMR signal mixed from the Larmor frequency to a 20 MHz carrier frequency for transmission to the receiver chain. This 20 MHz signal was routed, under pulse sequence control, to a gating device or the scanner digitizer within each TR. The gating device consists of a demodulation stage to remove the 20 MHz carrier frequency leaving the audio-frequency NMR signal in vector power mode. This signal was low-pass filtered (3.5 Hz, -3dBm) to reduce broadband noise and streamed to give a pseudo-continuous analogue CR signal to a standard physiological gating controller (Biopac MP150, DA100C amplifier and DTU200 gating unit). The DA100C amplifier included a 10 Hz low pass filter to generate the NMR derived signal trace labelled ‘NMR’ in Figure 1. The threshold level LR is combined with a user variable delay τ to generate the respiratory R-logic signal gate. The R-logic gate is used for selection of CR logic signals generated by the threshold level LCR such that only those heartbeats not coincident with the breaths were used for imaging. A steady-state maintained 2D gradient echo CINE scan (TE 3 ms, TR 5.3 ms, FA 10°, FOV 32x32 mm2, matrix 128x128, THK 1.5 mm, CINE loop NE 23) was operated with gating control and reacquisitions as described previously6 and shown in Figure 2. Two mice were anaesthetized (isoflurane 1-2% in 30% oxygen in air) twice each for imaging. Subcutaneously-implanted ECG needles and a pressure balloon were used to assess the conventional prospectively ECG-gated scan mode and to monitor the animal whilst MRI was not being performed. The ECG and pressure balloon signals were then removed from the gating controller and replaced with the NMR signal.Results
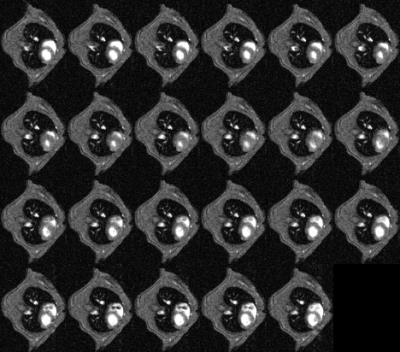
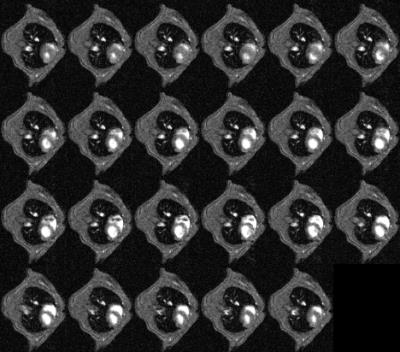
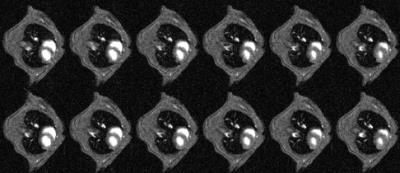
CINE images produced using conventional prospective ECG-driven gating and with the prospective self-gating are shown in Figures 3 and 4, respectively. A clear delay in the onset of imaging was observed for the self-gated scan consequent to the choice of prototype audiofilter used to suppress noise on the NMR-derived physiological signal trace. Figure 5 shows that when images from end diastole onwards are aligned for each scan mode the images are near equivalent, though there is some additional blurring in the self-gated images. The prospectively self-gated CINE images took approximately 30 s to acquire. The traces for the ECG, respiration and NMR signals of Figure 1 are taken from one scan for one mouse and were observed to be representative for all scans.Discussion
CINE imaging in the mouse, driven through prospective self-gating was straightforward to implement without any significant modification of the basic scanner hardware beyond that required for conventional prospective gating. The additional signal processing hardware acted as a bridging point between the NMR receiver line and the commercial gating control unit. Whilst the filtering used to suppress the noise on the NMR signal used to control gating was harsh, with a rather significant delay to the onset of imaging, this can be improved upon through use of a better-designed filter. The blurring seen in the self-gated scan is, we believe, a result of the increasing baseline signal seen in between breaths causing a jittered alignment of phase encode steps with the cardiac cycle. Again, this can be improved upon with a better signal filtering or conditioning scheme, such as gating via the derivative of the cardiac signal. The time saving enabled by using prospective self-gating compared to retrospective self gating is quite limited in the case of CINE imaging described, but would be much higher in the case of k-space segmented acquisitions in which multiple lines of k-space can be acquired in an ordered manner after detection of each selected cardiac event.
Conclusion
Prospective self-gated imaging is straightforward to implement, even for mice, and offers the potential to reduce the scan times required for retrospectively self gated imaging.Acknowledgements
The work presented was supported financially by Cancer Research UK (CRUK grants C5255/A12678, C2522/A10339), the Engineering and Physical Sciences Research Council (EPSRC grant C2522/A10339) and the Medical Research Council Unit Grant for the Oxford Institute for Radiation Oncology.References
1. Kim WS, Mun CW, Kim DJ, Cho ZH. Extraction of cardiac and respiratory motion cycles by use of projection data and its applications to NMR imaging. Magn Reson Med. 1990. 13(1):25-37.
2. Bishop J, Feintuch A, Bock N, Nieman M, Dazai J, Davidson L and Henkelman RM. Retrospective Gating for Mouse Cardiac MRI Magnetic Resonance in Medicine 55:472–477 (2006)
3. Esparza-Coss E, Ramirez MS, and Bankson JA. Wireless Self-Gated Multiple-Mouse Cardiac Cine MRI Magn Reson Med. 2008 59(5): 1203–1206.
4. Hiba B, Richard N, Janier M, Croisille P. Cardiac and respiratory double self-gated cine MRI in the mouse at 7 T. Magn Reson Med. 2006. 55(3):506-13.
5. Buehrer M, Curcic J, Boesiger P, Kozerke S. Prospective self-gating for simultaneous compensation of cardiac and respiratory motion. Magn Reson Med. 2008 60(3):683-90.
6. Kinchesh P, Gilchrist S, Gomes AL, Kersemans V, Beech J, Allen D, Smart S. Accelerated Imaging of the Mouse Body using k-space Segmentation, Cardio-Respiratory Synchronisation and Short, Constant TR: Application to b-SSFP. Proc Intl Soc Mag Reson Med 2016;24:1825.
Figures