1282
A Retrospective Cardiac Gating Method using Simultaneously Acquired Navigator1Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of
Synopsis
A cardiac gating method using a navigator MR signal requires the additional acquisition steps interleaved with the acquisition of image signals. To eliminate the additional acquisitions for navigators, the proposed method obtains an image signal and a navigator signal at the same time, and separates them in post-processing. Cardiac motion information estimated from the separated navigators is used for retrospective cardiac gating. To demonstrate the feasibility of the proposed method, in-vivo experiments were performed for cardiac MRI with short axis view.
Introduction
In order to handle cardiac motions, several methods employ the MR signals as navigators for motion synchronization of data acquisitions1-3. Among the various types of navigators, the projection-based navigators are appropriate for cardiac MRI, because they provides spatial-discriminant information and high temporal resolution for motion monitoring1. However, in the Cartesian k-space trajectory, the projection-based navigators require additional acquisitions, which are interleaved of acquisitions for imaging data. This procedures decrease the imaging time efficiency. In this abstract, a simultaneously acquired navigator technique is proposed for cardiac motion correction while maintaining high imaging time efficiency in the Cartesian trajectory.Methods
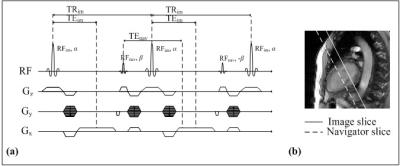
The proposed method simultaneously acquires an image signal and a navigator signal, and separates them in post-processing steps. The proposed pulse sequence is depicted in Figure 1. The conventional SSFP pulse sequence is modified to add an additional RF pulse, whose duration is very short (0.13 ms). The additional RF pulse excites a supplementary slice for navigator signal. The excited navigator signal is rephased with an image signal at the next refocusing readout gradient. This procedures enable to obtain navigator signals while maintaining the echo time (TE) of image signals, thereby slightly increasing the repetition time (TR). The acquired k-space data (Sim+nav) is the summation of image signals and navigator signals, where the navigator signal is not affected by the phase encoding gradient.
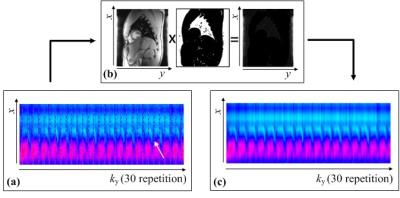
In post-processing steps, the navigator signals (Snav) and image signals (Sim) are estimated from the acquired data, respectively. When there is no motion in the navigator slice, the navigator signals of Sim+nav are identical along the phase-encoding dimension (ky), where its 1D Fourier-transform in the phase encoding direction makes the navigators to be a line signal at center of phase encoding dimension (y). By using the alternating RF phases of 0° and 180°, the Fourier-transformed navigator signals are located at the boundary of y, where the image signals are usually null. Even though the object has cardiac motions, navigator signals can be spatially discriminable with image signals in spatial domain, if TR is much shorter than the cardiac period. Therefore, Snav for motion monitoring can be extracted from the Sim+nav by using a mask operation in spatial domain, where the spatial information of image signals is zero. To make the mask, the data consisting only of image signals is acquired before the acquisition for cardiac imaging. The mask operation is depicted in Figure 2.
Because the navigator signals of adjacent phase-encoding steps are very similar except the opposite phases, the summation operation between adjacent phase-encoding lines of Sim+nav makes the navigator signals to be null as follows:
$$S_{im}(k_{x},n)=\begin{cases}\frac{1}{2}(S_{im+nav}(k_{x},n)+S_{im+nav}(k_{x},n+1)) & n = 1,...N_{pe}-1\\0 & n =N_{pe}\end{cases}, \tag1$$
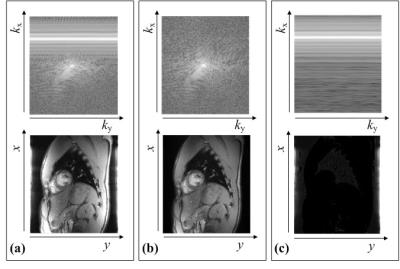
where kx is the readout dimension of k-space, and Npe is the total number of phase-encoding points. After this operation, even though image signals are weighted by a window in spatial domain, where the window has a shape of the main lobe of 1D sinc curve along the y direction, this effect can be compensated for in spatial domain. Therefore, Sim can be extracted from Sim+nav without loss of the image components. From Snav and Sim, the motion-gated cardiac images can be obtained by the navigator-based reconstruction method4. The k-space and spatial information of Sim+nav, Sim, and Snav are shown in Figure 3.
In-vivo experiments were performed using a 3.0T MRI system (Siemens MAGNETOM Verio, Erlangen, Germany). Cardiac imaging with short axis was performed using the following parameters: FOV = 263×350 mm2, matrix size = 144×192, TR/TE = 3.5/1.6 ms, slice thickness = 8(image)/16(navigator) mm, flip angle = 10(α)/2(β) °, and retrospective reconstruction. During the breath-hold, 30 full k-space data were acquired.
Results
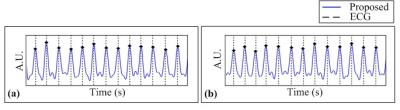
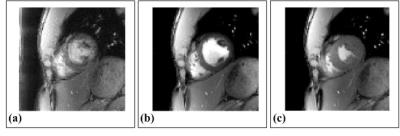
In Figure 4, the estimated cardiac motions are shown as solid blue lines and dots with the reference signals depicted as dotted lines, which are from the ECG device. The cardiac trigger points of the proposed method is highly synchronized with that of ECG devices. The results of cardiac imaging with short axis view are shown in Figure 5. The navigator components are clearly eliminated in motion-gated images. None of the results contain motion artifacts.Conclusion & Discussion
The proposed method provides the projection-based navigators for cardiac motion monitoring while maintaining the high imaging time efficiency. In-vivo experiments demonstrated that the simultaneously acquired image signals and navigator signals could be separated by the simple post-processing steps. The results showed that the proposed method could be a reliable tool for cardiac MRI.Acknowledgements
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014M3C7033999).
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI14C1135)
References
1. Kim W, Min C, Kim D, et al. Extraction of cardiac and respiratory motion cycles by use of projection data and its applications to NMR imaging. Magn Reson Med 1990;13:25–37.
2. Crowe ME, Larson AC, Zhang Q, et al. Automated rectilinear self-gated cardiac cine imaging. Magn Reson Med 2004;52:782–788.
3. Larson AC, White RD, Laub G, et al. Self gated cardiac cine MRI. Magn Reson Med 2004;51:93–102.
4. Odille F, Uribe S, Batchelor PG, et al. Model-based reconstruction for cardiac cine MRI without ECG or breath holding. Magn Reson Med. 2010;63:1247-1257.
Figures