1278
Automatic reference-free detection and quantification of MR image artifacts in human examinations due to motionThomas Küstner1,2, Annika Liebgott1,2, Lukas Mauch2, Petros Martirosian3, Konstantin Nikolaou1, Fritz Schick3, Bin Yang2, and Sergios Gatidis1
1Department of Radiology, University of Tuebingen, Tuebingen, Germany, 2Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 3Section on Experimental Radiology, University of Tuebingen, Tuebingen, Germany
Synopsis
MRI has a broad range of applications due to its flexible acquisition capabilities. This demands profound knowledge and careful parameter adjustment to identify stable sets which guarantee a high image quality for various and, especially in examinations of patients, unpredictable conditions. This complex nature and long examination times make it susceptible to artifacts which can markedly reduce the diagnostic image quality. An early detection and possible correction of these artifacts is desired. In this work we propose a convolutional neural network to automatically detect, localize and quantify motion artifacts. Initial results in the head and abdomen demonstrate the method’s potential.
Purpose
MRI is
a versatile imaging modality which allows the precise and non-invasive assessment
of anatomical structures and pathophysiological pathways. A flexible sequence
and reconstruction parametrization make it very tunable to specific
applications to meet the demanded diagnostic requirements. These quality
criteria and the respective parametrizations need to be optimized and ensured
to provide sufficient quality for changing acquisition conditions often without
the availability of reference data. The relatively long examination times and
varying acquisition conditions predispose it to imaging artifacts which may
result in a deterioration of diagnostic image quality. Artifacts can originate
from hardware imperfections, signal processing inaccuracies and, in most cases,
from the compliance and behavior of the patient which can demand a re-scan if
the image is too severely affected by e.g. motion. However, the quality with
respect to the diagnostic application is often just checked retrospectively by
manual reading of a human observer (HO) when the patient has already left the
facility, i.e. a re-scan is just possible with larger effort in an additional
exam. Careful operation together with prospective or retrospective correction
techniques1,2 may reduce artifact burden, but are either not always
applicable and/or cannot correct for all different types of induced artifacts. It
is therefore desired to have an automatic image quality assessment directly
after the acquisition with feedback of the acquired image quality and a suitable
sequence re-parametrization suggestion to the operating technician. We
therefore proposed a non-reference MR image quality assessment (IQA) system
based on a machine-learning approach to predict HO labeling scores of arbitrary
input images corrupted with unknown artifacts3,4,5. A reliable
quality prediction depends on the derived features. In this work, we will therefore
focus on the automatic detection and quantification of motion artifact examples
of rigid head motion and non-rigid respiratory motion in the thorax. Motion
detection methods aim to track the underlying motion by an MR navigator6,7,8
via external sensors9,10,11 or from the acquired time-series images,
e.g. via registration12,13. But no motion quantification or
localization has been conducted up to now. We propose the usage of a
convolutional neural network (CNN) to quantify and localize motion artifacts
for IQA.Material and Methods
The proposed CNN is shown in Fig.1. The CNN consists of three convolutional layers for the detection and localization of the artifacts. A subsequent fully connected multilayer perceptron with a sigmoid activation function feeds two soft-max output nodes for a probabilistic quantification p of the artifact burden. Input images are patched into sizes of 40x40 overlapping patches to enable the localization and to reduce the computational complexity respectively the number of parameters to be trained. Each convolutional layer consists of N filter kernels with size MxLxB, a rectifying linear unit activation function and a downsampling layer. A dyadic increase in the filter kernel depth N provides a multi-resolution approach. Filter coefficients and bias values are trained via a steepest gradient descent. Parameter optimization and training is conducted by means of a 10-fold cross validation on 10 volunteer datasets (3 female, age 27±4.5 years). Datasets are acquired for each volunteer on a whole-body 3T PET/MR (Biograph mMR, Siemens) with a T1w-TSE in the head and a T1w-GRE in the abdomen. For each region, two datasets are acquired with and without motion artifacts (head: rigid head tilting, abdomen: non-rigid respiratory movement) which serve directly as labels for the supervised training.Results and Discussion
Fig.2 and 3 display the acquired images of one subject with and without motion artifacts in the head and abdomen, respectively, with a colored overlay of the probability map p for the patches. It can be seen that the artifact affected regions are correctly localized and show a high probability of motion artifacts. For all subjects an average test accuracy of 85% in the head and 70% in the abdomen was achieved. A detection of motion is thus possible in both regions with a superior performance in the head region, because of the more systematic artifact manifestation in the image. These initial results indicate the potential feasibility, but the study has its limitations. So far no patch-based labeling (motion-free and motion-affected) was conducted and the artifact detection was just targeted on motion artifacts on a specific sequence of a single anatomic region.Conclusion
We propose an algorithm to detect, localize and quantify motion artifacts in the head and abdominal region for a T1w sequences. Future studies will investigate the reliability and robustness to transfer the results to other sequences, body regions and artifacts. This would enable the usage as a reliable automatic predictor in the context of an automatic IQA.Acknowledgements
No acknowledgement found.References
[1] McClelland et al., Med Image Anal 2013:17(1). [2] Godenschweger et al., Phys Med Biol 2016:61(5). [3] Küstner et al., Proc ISMRM 2015. [4] Küstner et al., Proc ISMRM 2016. [5] Liebgott et al., Proc ICASSP 2016. [6] Ehman and Felmlee, Radiology 1989:173. [7] Fu et al., MRM 1995:34. [8] Ward et al., MRM 2000:43. [9] Derbyshire et al., JMRI 1998:8. [10] Zaitsev et al., Neuroimage 2006:31. [11] Maclaren et al., PLoS One 2012:7. [12] Kim et al., IEEE TMI 2010:29. [13] Metz et al., Med Image Anal 2011:15.Figures
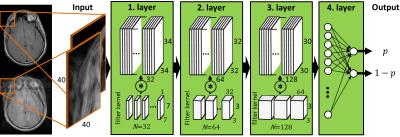
Fig. 1: Proposed
convolutional neural network with three hidden layers and one fully-connected
output layer to estimate artifact probabilities p on a patch-basis.
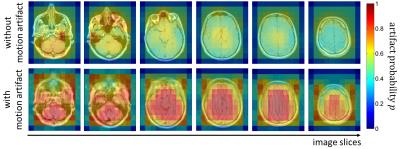
Fig. 2: Probability
map p for artifact occurrence on
patch-basis overlaid to T1w TSE image of the head in different slice locations. Top
row: images acquired without artifact (lying still). Bottom row: images
acquired with artifact (head tilding in left-right direction).
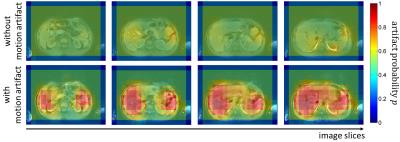
Fig. 3: Probability
map p for artifact occurrence on
patch-basis overlaid to T1w GRE abdominal image in different slice locations.
Top row: images acquired without artifact (breath-hold). Bottom row: images
acquired with artifact (free-breathing).