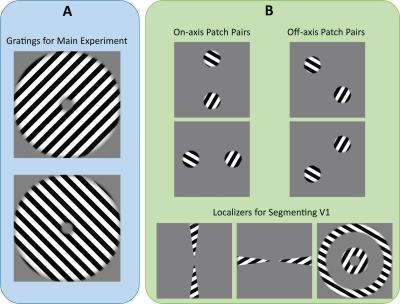
1276
Quantifying the effectiveness of Prospective Motion Correction using a Visual fMRI task1Cognition and Brain Science Unit, MRC, Cambridge, United Kingdom, 2Department of Systems Neuroscience, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany
Synopsis
Prospective Motion Correction (PMC) using an optical tracking system has been shown to improve data quality. We conducted a study on 18 subjects using robust visual stimuli to quantify the effectiveness of PMC on task-based fMRI. Our results show that PMC improves voxel-to-voxel registration across time and leads to better contrast-to-noise ratio. This is particularly evident in analyses which are more sensitive to inaccurate voxel registration and motion-induced noise.
Introduction
The visual cortex has been shown to demonstrate a strong spatial activation pattern in response to gratings of different orientations1–4. In this study, we attempt to utilize this to quantify the effects of Prospective Motion Correction (PMC) on task-based fMRI data in the visual cortex. While PMC has been shown to greatly benefit structural scans5, much less is known about the effects of PMC on task-based fMRI.Methods
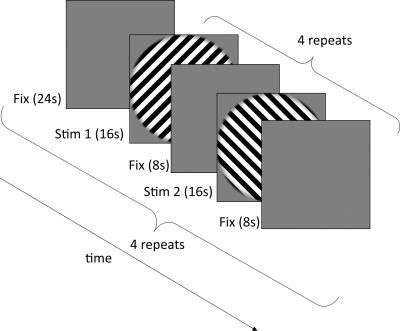
A study was conducted on 18 subjects. The subjects were required to be present for 3 separate scanning sessions: (Condition 1) with motion correction and mouthpiece on, (Condition 2) with the mouthpiece on but motion correction switched off and (Condition 3) with no mouthpiece. In all cases, subjects were instructed to remain as still as possible. Subjects were imaged at 2 different resolutions, 3mm and 1.5mm, followed by a localizer scan. The session order and the scanning order for each resolution within sessions were randomised across subjects. Throughout all experiments, subjects were asked to fixate on a blue dot in the centre of a screen and to press a button whenever the dot flashes green. Subjects with task accuracy lower than 50% were excluded from further analysis.
All data was collected on a 3T Siemens Prisma with a 32-channel head coil. Imaging parameters for the 3mm isotropic EPI were: TR=1260ms TE=30ms FA=78o Matrix size=64*64*20 ToA=~11mins. Imaging parameters of the 1.5mm isotropic EPI were: TR=3050ms TE=30ms GRAPPA=2 FA=78o Matrix size=128*128*40 ToA=~11mins. Motion data was acquired using a commercially available optical PMC system (KinetiCor Inc, HI, USA) which tracks a single Moire phase marker. The marker was attached to a custom-moulded mouthpiece for each subject. Data was analysed in SPM 8. Extraction of V1 and retinotopic ROIs was done in Freesurfer 5.3.0. The timings for the stimulus presentation are described in figure 2. A support vector machine (SVM) classifier was used to learn and classify the regressor patterns for the two different orientations.
For experiment 1, the SVM was trained on all the voxels from V1.
For experiment 2, retinotopic masks of different localizer patch pairs were generated to control for radial bias2. The presence of radial bias in off-axis patch pairs results in retinotopic maps with lower spatial frequency when compared to on-axis patch pairs. Hence, classification using ROIs from off-axis patch pairs should be robust against small fluctuations in motion while ROIs from on-axis patch pairs would be sensitive to any motion that results in improper registration.
For experiment 3, the SVM classifier was executed using the N-most discerning voxels in V1 for each training set. By using a small number of voxels, classification accuracy would serve as a strong indicator for accuracy of voxel-to-voxel registration across time. Moreover, high classification accuracy for this classifier would indicate a clear and strong contrast as the most discerning voxels are being reliably identified.
Results
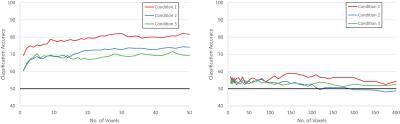
The results for experiment 1 and 2 are shown in Figure 3 while the results for experiment 3 are shown in Figure 4.
Experiment 1
At 3mm, applying PMC (Condition 1) shows a slight increase in classifier accuracy. At 1.5mm, the presence of the mouthpiece (Condition 2) caused a clear decrease in classifier accuracy but this is counteracted by the application of PMC (Condition 1). This suggests that there is an increase in motion due to the presence of the mouthpiece.
Experiment 2(off-axis patch pairs)
At 3mm, the classification accuracy is relatively similar to that of V1 while at 1.5mm, using the off-axis patch pairs performs better than V1. This agrees with the literature that decoding in V1 is strongly driven by radial bias3,4.
Experiment 2(on-axis patch pairs)
Due to the higher spatial selectivity when no radial bias is present, there is a drop in classification accuracy across all conditions as compared to V1. This drop is the smallest when PMC is applied (condition 1) for both resolutions. Comparing within resolutions for on-axis patch pairs, applying PMC (condition 1) outperforms the other two conditions. This suggests that PMC helps to preserve the fine-grained activation patterns.
Experiment 3
Below 50 voxels for 1.5mm, the difference in performance of the three conditions is indiscernible due to the noise present. In all other cases, applying PMC (condition 1) consistently outperforms the other two conditions.
Discussion and Conclusion
Our analysis has shown that PMC improves the quality of the data by enhancing the contrast-to-noise ratio for the data and improving voxel-to-voxel registration. This provides strong evidence that high resolution fMRI would greatly benefit from using PMC to improve image quality.Acknowledgements
MRI scans were funded by the Medical Research Council (MRC), UK. Pei Huang is also funded by the Agency of Science, Technology and Research (A*STAR), Singapore.References
1. Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8(5):679-685. doi:10.1038/nn1444.
2. Tong F, Swisher JD, Gore JC, et al. Multiscale Pattern Analysis of Orientation-Selective Activity in the Primary Visual Cortex. J Neurosci. 2010;30(1):325-330. doi:10.1523/JNEUROSCI.4811-09.2010.
3. Freeman J, Heeger DJ, Merriam EP. Coarse-scale biases for spirals and orientation in human visual cortex. J Neurosci. 2013;33(50):19695-19703. doi:10.1523/JNEUROSCI.0889-13.2013.
4. Alink A, Krugliak A, Walther A, Kriegeskorte N. fMRI orientation decoding in V1 does not require global maps or globally coherent orientation stimuli. Front Psychol. 2013;4(AUG). doi:10.3389/fpsyg.2013.00493.
5. Stucht D, Danishad KA, Schulze P, Godenschweger F, Zaitsev M, Speck O. Highest resolution in vivo human brain MRI using prospective motion correction. PLoS One. 2015;10(7):1-17. doi:10.1371/journal.pone.0133921.
Figures