1269
Automated pre-processing pipeline and quality control for neonatal diffusion MRI in the developing Human Connectome Project (dHCP)1Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, Oxford, United Kingdom, 2Centre for the Developing Brain, King’s College London, London, United Kingdom, 3Department of Computing, Imperial College London, London, United Kingdom
Synopsis
The developing Human Connectome Project (dHCP) is a collaborative 6-year project set to create a 4-dimensional map of structural and functional changes occurring throughout early development. Up to 1300 multi-modal MRI scans of foetuses and neonates (20 to 44 weeks gestational age) are currently being acquired. We present a fully automated pre-processing pipeline that allows us to efficiently analyse in-vivo diffusion MRI (dMRI) data despite the considerable technical challenges specific to neonatal imaging. We developed a quality control (QC) framework that allows us to identify issues or inconsistencies. This is especially useful when processing a very large number of subjects.
Purpose
The developing Human Connectome Project (dHCP) is set to create and make openly available to the scientific community a 4-dimensional map of early structural and functional developmental changes. Up to 1300 multi-modal MRI scans from foetuses and neonates (20 to 44 weeks gestational age) are currently being acquired, along with genetic, clinical and developmental information. In this overview, we focus on diffusion MRI (dMRI) image processing and the structural connectivity aspect of the project. We present a fully automated pre-processing pipeline and a quality control (QC) framework that allow us to efficiently analyse in-vivo data despite the considerable technical challenges specific to neonatal imaging (e.g., small size, changing tissue contrast, excessive motion). The data quality described here is representative of some initial datasets that will be released to the community in early 2017.Methods
A highly optimized sequence (Multiband factor of 4, 4 phase encode (PE) directions, 20 b0s, 400-1000-2600s/mm2 b-values, 64:88:128 dMRI volumes, 1.5x1.5x3mm voxels, -1.5mm slice gap to allow super-resolution, 64 slices, 4000/90ms TR/TE) developed for scanning neonates during natural sleep has been implemented on a clinical 3T Philips Achieva scanner equipped with a dedicated 32-channel neonatal head coil and a patient handling system1,2.
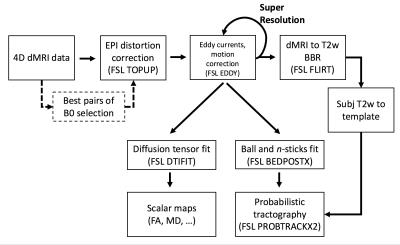
Pre-processing: A significant challenge in analysing neonatal dMRI data is dealing with a high degree of motion, leading to both misalignments and unusable data due to large signal dropout. Firstly, the pre-processing pipeline (Fig. 1) automatically selects the b0 pairs least-affected by intra-volume motion for each PE direction to optimally estimate fieldmaps for correcting susceptibility distortions3. Then, a comprehensive framework, based on a Gaussian process data predictor (FSL EDDY), simultaneously estimates subject motion and eddy currents4,5. Using the estimated distortion fields, the framework detects and replaces outlier slices in distorted (i.e., uncorrected) space6. These, together with slice-to-volume alignment, are crucial steps when dealing with high levels of intra-volume motion. Furthermore, they create the best initial conditions for a subsequent super-resolution algorithm7, which is applied along the slice-selection direction, to achieve high isotropic resolution of 1.5mm. The super-resolved data are then fed back to EDDY, which now corrects for all distortions simultaneously. Diffusion data is then aligned to structural (T2-weighted) space using a boundary-based-registration approach8,9 that relies on the contrast difference between white and grey matter in the average attenuation volume for the b=1000s/mm2 shell. This is combined with a non-linear registration10 of the T2w to a neonatal longitudinal atlas11 to allow transformations between diffusion and atlas spaces.
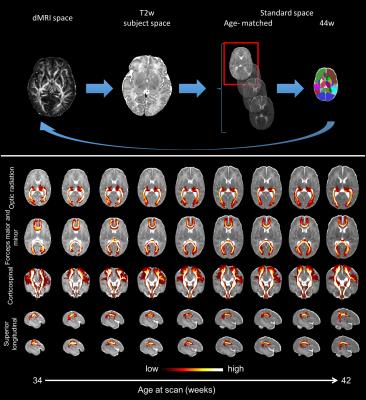
Automated probabilistic tractography: Given the significant structural changes that occur in the neonatal brains, it is challenging to build a standardized atlas of white matter connections. We have implemented an automated probabilistic tractography pipeline to segment 30 main association and commissural tracts in age-matched template space12. This uses fibre orientations estimated from multi-shell data using a model-based approach13,14 that takes into account the inherent low anisotropy of neonatal white matter and performs parametric spherical deconvolution with an axially symmetric tensor impulse response. Crucially, the pipeline uses non-linear transformations to warp a set of standardized ROIs to the age-of-scan-matched template10,11. The tool can easily be extended/modified and is computationally efficient using GPUs15.
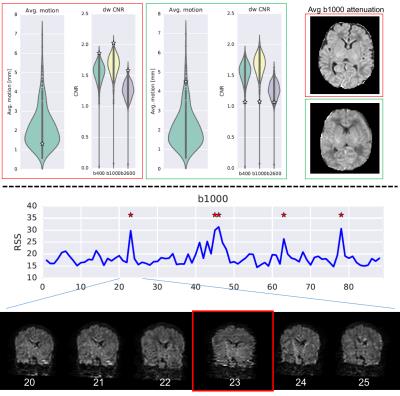
Quality control: The pipeline automatically generates QC indices reflecting: overall dataset information (complete/usable), outliers (e.g., outliers per volume/shell), contrast and signal to noise ratios, absolute subject motion and quality of the registration. A Python-based framework automatically generates two types of reports (whole population and single subject level).
Results
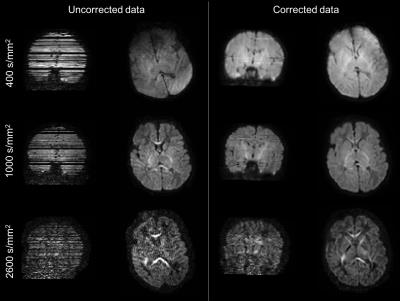
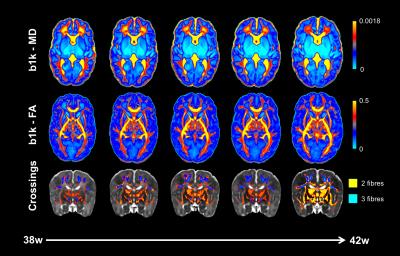
Fig. 2 shows example volumes for each b-shell before and after distortion/motion correction. The overall brain geometry and outlier slices affected by large signal dropouts in volumes with high degrees of motion are restored. Fig. 3 shows the expected overall increase in anisotropy across age, with different areas showing different fractional anisotropy (FA) and mean diffusivity (MD) trajectories. Fibre crossings maps show an overall increase in structural complexity across age, with more voxels showing two and three fibre populations across age. Fig. 4 shows the automated tractography pipeline and the results obtained when selecting a subset of tracts across different ages. Fig. 5 shows example QC that allow us to automatically identify possible issues within a dataset and rank a subject w.r.t. the group.Conclusions
We presented an automated pipeline to pre-process neonatal dMRI data that is capable of providing high-quality results and can effectively deal with the main challenges presented by neonatal data. We are working towards extending the pipeline with the ultimate aim of linking structural connectivity profiles and microstructural information to genetics and behaviour to better understand how development of structural-functional interactions influence each individual.Acknowledgements
We are thankful to our colleagues from the dHCP recruitment, radiography and research nurse team. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013) / ERC Grant Agreement no. 319456.References
1. Hughes, E. J. et al. A dedicated neonatal brain imaging system. Magn Reson Med (2016).
2. Tournier, J. D. et al. Data-driven optimisation of multi-shell HARDI in Annual meeting of the ISMRM. Toronto, Canada, 2015.
3. Andersson, J. L., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870-888 (2003).
4. Andersson, J. L. & Sotiropoulos, S. N. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. Neuroimage 122, 166-176 (2015).
5. Andersson, J. L. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063-1078 (2016).
6. Andersson, J. L., Graham, M. S., Zsoldos, E. & Sotiropoulos, S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141, 556-572 (2016).
7. Kuklisova-Murgasova, M., Quaghebeur, G., Rutherford, M. A., Hajnal, J. V. & Schnabel, J. A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Medical Image Analysis 16, 1550-1564 (2012).
8. Greve, D. N. & Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63-72 (2009).
9. Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825-841 (2002).
10. Andersson, J. L. R., Jenkinson, M. & Smith, S. Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2. (2010).
11. Serag, A. et al. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. NeuroImage 59, 2255-2265 (2012).
12. de Groot, M. et al. Improving alignment in Tract-based spatial statistics: Evaluation and optimization of image registration. NeuroImage 76, 400-411 (2013).
13. Jbabdi, S., Sotiropoulos, S. N., Savio, A. M., Grana, M. & Behrens, T. E. Model-based analysis of multishell diffusion MR data for tractography: how to get over fitting problems. Magn Reson Med 68, 1846-1855 (2012).
14. Sotiropoulos, S. N. et al. Fusion in diffusion MRI for improved fibre orientation estimation: An application to the 3T and 7T data of the Human Connectome Project. NeuroImage 134, 396-409 (2016).
15. Hernandez-Fernandez, M. et al. A fast and flexible toolbox for tracking brain connections in diffusion MRI datasets using GPUs in Annual meeting of the OHBM. Geneva, Switzerland, 2016.
Figures