1262
Golden-Angle Spiral Sparse Parallel Phase-Ccontrast MR acquisition with an on-line fast GPU based reconstruction for high resolution real-time cardiovascular assessments1Institute of Cardiovascular Science, University College London, London, United Kingdom, 2Great Ormond Street Hospital for Children, London, United Kingdom
Synopsis
Compressed Sensing for 2D spiral PCMR with golden angle acquisition schema.
The work presents a significant improvement in spatial-temporal resolution of the real-time spiral PCMR data, which are comparable with the standard high resolution cardiac gated sequences. The technique proved to be suitable for clinical use with the benefits of short acquisition times and no breathing artefacts.
PURPOSE
Assessing flow using PCMR is useful in patient with congenital heart disease. However, conventional free breathing flow acquisitions (with signal averaging) are time consuming, whilst breath holds sequences are often low resolution. Real-time PCMR offers a solution but requires high level of acceleration. Therefore, we have developed a Golden-Angle Spiral Sparse Parallel (GASSP) PCMR acquisition with an on-line fast GPU based reconstruction. The developed CS reconstruction utilises the wavelet transform in space and finite difference operator in space and time domains as the sparsifiying transforms. The aim of this work is to validate this sequence in patients.METHODS
The study population consisted of 16 older children and adults (3 feamals and 13 males; aged: 11-54, median: 30) with congenital heart disease. The uniform density spiral PCMR real-time sequence(1) (voxel: 2.0x2.0x7.0mm, TR/TE: 6.63/1.85ms, 30 interleaves for fully sampled k-space, VENC=200cm/s, B0=1.5T) was modified to acquire Golden-angle data. The single spiral interleave was rotated by ~222o with each read-out. Two consecutive read-outs constituted a single frame resulting in 15x acceleration and temporal resolution of 26.52ms. A conventional Cartesian gated PCMR was acquired as the gold-standard reference (voxel: 1.8x1.8x7.0mm, TR/TE: 19.45/2.08ms, VENC=200cm/s, NSA: 3) with two times in phase direction reduction (GRAPPA). 180 frames (~4.8s) of the new sequence were acquired directly before and after the reference scan (on average ~152s). The GASSP data was reconstructed on-line(2) on an external computer equipped with Tesla C2070, NVIDIA graphics card (GPU). A GPU based SENSE reconstruction(3) was changed to a non-linear conjugate gradient solver for CS L1 regularisation problems(4). The solving process was fixed to 35 solver iterations as a compromise between sufficient artefact suppression and reconstruction time. The Golden-angle strategy generates the incoherent aliasing in the time domain. Consequently, a sparse transform needs to be applied to the time component of the data. Our experiments showed Finite difference operator to significantly outperform Fourier transform as the temporal sparsifier (evaluated by visible residual artefacts). Additionally, five level Haar transform and Total Variance (Finite difference in space) were added as L1 regularisations to aid the artefact and noise suppression. The regularisation parameters were selected empirically.
The continuous data was reconstructed in blocks of 60 frames. As a counter measure to potential jump discontinuations between blocks these were expanded to overlap each other (six frames from the adjacent blocks). If there was no adjacent block (the first and last blocks) the expansion was done by mirroring of frames.
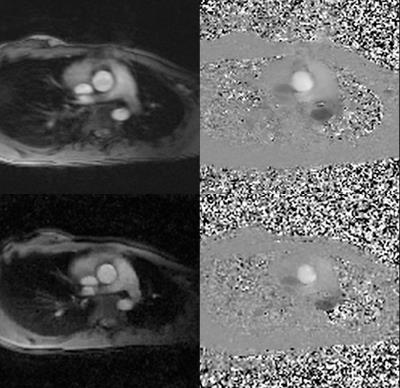
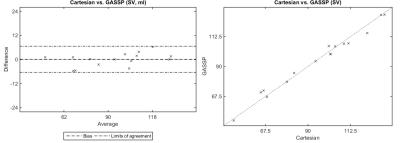
The imaging position was set to the ascending aortic view. The resultant images were segmented semi-automatically(5) and processed using in-house plugins for Osirix software(6). Flow validation was done on a base of Stroke Volume (SV) data. The GASSP results were averaged and compared to the gold standard using Bland-Altman and correlation analysis.
RESULTS
GASSP PCMR was successfully acquired in all patients. The GASSP reconstruction required ~2min on average to finish. Fig. 1 presents imaging results. Bland-Altman (Fig. 2) analysis showed no significant differences (bias: 0.16±3.32ml, limits of agreement: -6.35 to 6.68 ml, p=0.85) in Stroke Volume (SV) measurements between the Gold-standard Cartesian (94.8±23.9ml, 50.7 to 130.5 ml) and GASSP (94.6±22.5ml, 49.5 to 129.0 ml). There was a good correlation between the measurements (r=0.99, p<0.001).DISCUSSION
GASSP reconstruction produced images with good image quality even with substantial undersampling. More importantly there was no bias in flow measurements between GASSP and the Gold-standard techniques with narrow limits of agreement.
The main limitation of the technique is its relatively long reconstruction time. This can be elevated by further implementation optimisations or splitting of the reconstruction among multiple GPUs.
CONCLUSION
We have validated a new CS reconstruction for PCMR spiral acquisitions. The work presents a significant improvement in spatial-temporal resolution of the real-time spiral PCMR data, which are comparable with the standard high resolution cardiac gated sequences. The technique proved to be suitable for clinical use with the benefits of short acquisition times and no breathing artefacts. We believe the new technique has a potential to be a valuable tool in cardiovascular assessments, particularly in small children.Acknowledgements
No acknowledgement found.References
1. Steeden JA, Atkinson D, Taylor AM, Muthurangu V. Assessing vascular response to exercise using a combination of real-time spiral phase contrast MR and noninvasive blood pressure measurements. J Magn Reson Imaging 2010;31(4):997-1003.
2. Kowalik GT, Steeden JA, Atkinson D, Taylor A, Muthurangu V. Implementation of a Heterogeneous Image Reconstruction System for Clinical Magnetic Resonance. Parallel Processing and Applied Mathematics. doi: Springer Berlin Heidelberg, 2014; 469-479.
3. Kowalik GT, Steeden JA, Pandya B, et al. Real-time flow with fast GPU reconstruction for continuous assessment of cardiac output. J Magn Reson Imaging 2012;36(6):1477-1482.
4. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58(6):1182-1195.
5. Odille F, Steeden JA, Muthurangu V, Atkinson D. Automatic segmentation propagation of the aorta in real-time phase contrast MRI using nonrigid registration. J Magn Reson Imaging 2011;33(1):232-238.
6. Rosset A, Spadola L, Ratib O. OsiriX: An Open-Source Software for Navigating in Multidimensional DICOM Images. J Digit Imagin 2004;17(3):205-216.