1253
Spiral-accelerated short-TE MRSI with B1-insensitive 1D-semiLASER localization and real-time motion correction at 7TPhilipp Moser1, Bernhard Strasser1, Lukas Hingerl1, Michal Povazan1,2, Gilbert Hangel1, Ovidiu C. Andronesi3, Borjan Gagoski4, Aaron T. Hess5, Dylan M. Tisdall6, Andre van der Kouwe3, Siegfried Trattnig1,2, and Wolfgang Bogner1
1High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Christian Doppler Laboratory for Clinical Molecular MR Imaging, Medical University of Vienna, Vienna, Austria, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 4Fetal-Neonatal Neuroimaging & Developmental Science Center, Boston Children's Hospital, Harvard Medical School, Boston, MA, United States, 5Centre for Clinical Magnetic Resonance Research, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom, 6Perelman School of Medicine, Department of Radiology, University of Pennsylvania, Pennsylvania, PA, United States
Synopsis
In vivo MRSI at 7T offers advantages compared to lower field strengths, in particular higher SNR and improved spectral resolution. However, spectral quality is often limited by strong B1+ inhomogeneities, motion-related artifacts and scanner instability related B0 field drifts. To overcome these limitations, we have developed a 1D-semiLASER 2D-spiral-encoded MRSI sequence with real-time motion monitoring/correction using 3D EPI-based navigators (vNavs). Besides sequence stability we show that accurate B1-insensitive excitation can be achieved throughout the slice until the border of the brain. Using 7T-optimized motion correction, high spectral and metabolic map qualities are feasible even in the presence of motion.
Introduction
In vivo MRSI at 7T offers advantages compared to lower field strengths (B0), in particular higher SNR and improved spectral resolution. However, especially at ultra-high fields, spectral quality is often limited by strong B1+ inhomogeneities, motion-related artifacts and scanner instability related B0 field drifts [1]. This leads to quantification bias, poor VOI localization, spatial encoding errors and spectral degradation [2]. To overcome these limitations, we have developed a 1D-semiLASER 2D-spiral-encoded MRSI sequence with real-time motion monitoring/correction using 3D dual-contrast EPI-based navigators (vNavs) for 7T. This promising approach combines the benefits of B1-insensitive localization with online motion correction and time-efficient data sampling. Real-time motion correction has been shown to significantly improve MRSI data acquisition with/without MEGA editing [3], but only at 3T [4]. Also, spiral-MRSI has so far only been shown for 3T. 1D-semiLASER localization is a new localization approach, to improve volume selection within stringent SAR limitations at 7T. The purpose of this study is to demonstrate the feasibility of B1-insensitive spiral-accelerated 1D-semiLASER MRSI using gradient modulated adiabatic pulses and vNav-based real-time motion correction optimized for 7T to obtain high spectral quality. Robust metabolic imaging during involuntary patient movement and field drifts is possible.Methods
Phantom and in vivo (n=5) measurements were performed on a Siemens Magnetom 7T whole-body MR scanner using a 32-channel receive coil array combined with a volume transmit coil. MUSICAL [5] was adapted to be applied with spiral-encoding and used to properly combine the signal of individual receive channels. Slice excitation and VOI localization was achieved by 1D-semiLASER using a 900 us slice selective SINC excitation pulse and one pair of B1-insensitive Gradient Offset Independent Adiabatic (GOIA) pulses (W16,4 modulation, 8 ms duration, 10 kHz bandwidth) refocusing a 12 mm slice (Fig.1). The minimum echo time (TE) of 22 ms was selected. Constant density spiral-encoding in the (kx,ky)-plane was used [6]. The field of view (FOV) of 200x200 mm² was subdivided into 64x64 voxels resulting in a nominal voxel size of 3.1×3.1×12 mm³. MRSI data were obtained with 1024 spectral points, 2040 Hz spectral bandwidth, two temporal, 30 angular interleaves, TR 2800 ms, 5 averages, 80° FA and TA 14:50 min. Real-time motion correction was performed using dual-contrast, dual-shot volumetric EPI navigators inserted prior to MRSI water suppression. The vNav protocol was optimized for 7T including implementation of fat suppression and improved ghosting artifact correction as well as the following protocol: TR 10 ms; TE1/TE2 3.8/4.8 ms; matrix 32×32; 20 slices; FOV 256×256×144mm³; 8mm isotropic resolution; bandwidth 4222 Hz/pixel; FA 2°. The total navigator duration including online calculation was 600 ms. Localization and spectral quality were evaluated qualitatively and quantitatively (i.e. SNR). Phantom measurements were performed to ensure stability, robustness and performance of the sequence. Also, the interleaved EPI scans were optimized with respect to echo train length, TE and resolution to ensure short navigator acquisition times while minimizing artifacts at 7T. Five volunteers were scanned with a protocol comprising three MRSI experiments: 1) stationary baseline, 2) motion without correction (NOCO) and 3) motion with correction (MOCO). The volunteers were trained to rotate their heads roughly ± 5º left-right every 3 min. NMRSCOPE-B was used to generate basis sets which were then processed by LCModel to created fitted spectra and metabolic maps.Results
The spiral-accelerated 1D-semiLASER MRSI sequence provided accurate localization and high spectral quality both in vivo and in phantoms. After vNav sequence optimization for 7T the quality of 3D EPI images was significantly improved (reduced geometric distortions, Nyquist-ghosting, and lipid artifacts) (Fig.2). The precise tracking of translation, rotation, B0 shim changes and frequency drift (Fig.3) enabled real-time motion correction and recovered corrupted data in the presence of head movement (Fig.4) which caused a significant SNR reduction of 12%. The NOCO scan had an average SNR of 5.9±3.2 compared to 6.6±2.0 for the static scan. Applying motion correction resulted in an average SNR of 6.4±2.5. By applying VOI updates each TR, comparable spectral and metabolic map qualities were achieved as in the static scan even in scans with prolonged scan time (Fig.4,5).Discussion/Conclusion
This study demonstrated that using slice-selective adiabatic refocusing via a 1D-semiLASER selection with spiral-accelerated 2D-MRSI is feasible at 7T and that it provides accurate B1-insensitive spatial excitation throughout the slice even towards the border of the brain. Combined with real-time motion correction the presented sequence is therefore a robust basis especially for longer and thus motion-sensitive experiments such as J-difference editing. The next step will be to use this approach for in vivo MEGA-editing of GABA and glutathione in the human brain at 7T.Acknowledgements
This study was supported by the FFG Bridge Early Stage Grant No. 846505 and NIH grant R00HD074649.References
[1] Truong, TK. et al., Proc. ISMRM 2170 [2] Bogner, W. et al., NeuroImage. 2014;88:22-31 [3] Bogner, W. et al., Neuroimage. 2014;103:290-302 [4] Hess, AT. et al., NMR Biomed. 2012;25(2):347-58 [5] Strasser, B. et al., NMR Biomed. 2013;26(12):1796-805 [6] Adalsteinsson, E. et al., Magn Reson Med. 1998; 39:889–898Figures
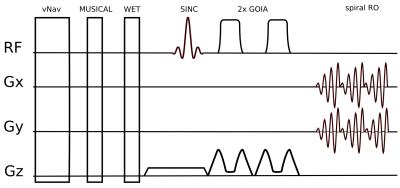
Figure 1: Sequence
schema of the short echo time 1D-semiLASER sequence. Slice selective
SINC excitation followed by one pair of adiabatic refocusing pulses,
generating a spin echo at 22 ms. vNavs are played out in each TR
before WET and MUSICAL prescan.
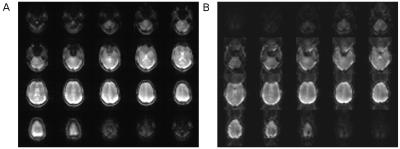
Figure 2: Rotational
and translational motion is calculated from magnitude images of 3D
EPI vNavs. (A) demonstrates sample images obtained from the
7T-optimized navigator protocol. While (A) shows strong suppression
of Nyquist-ghosting and distortions, the EPI images in (B) show
significant artifacts with the protocol used at 3T.
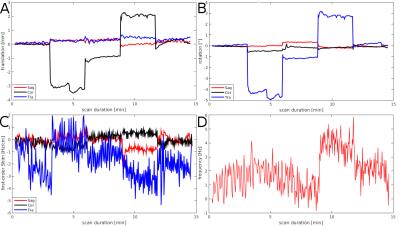
Figure 3: (A)
Translation, (B) rotation, (C) first-order shim terms, and (D)
frequency changes, as measured by the vNav during MRSI scan are
plotted as a function of scan time in the scanner coordinate system.
During the ~15min scan,
the volunteer
rotated (~5°) his head every 3 min in the following schema: right,
middle, left, middle. (D) shows very little non-motion related
scanner frequency drifts over 15 min, even with gradient-demanding
spiral RO.
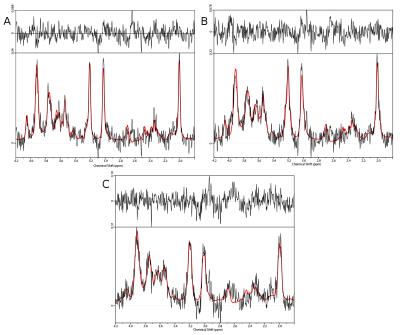
Figure 4: Sample
spectra from the central voxel. (A) shows the static scan without
intentional volunteer motion, the corresponding MOCO and NOCO spectra
are shown in (B) and (C), respectively. While (C) clearly shows
motion-related spectral degradation (i.e. less SNR, broader
linewidths), applying motion correction in (B) could recover the
spectral quality.
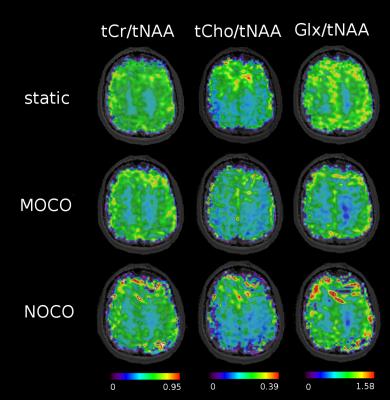
Figure 5: Metabolic
ratio maps for one sample volunteer. The NOCO maps show overall
reduced signal distribution with signal deficits on the border of the
brain compared to the static scans. MOCO could recover metabolic map
quality, however there's still room for improvements especially with
respect to online shim and frequency updates, which are highly
sensible at 7T.