1247
Three-dimensional 31P Radial Echo-Planar Spectroscopic Imaging In Vivo at 7T1Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
31P MR spectroscopic imaging (31P MRSI) in vivo suffers from low spatial resolution and long measurement times. The purpose of this study was to prove feasibility of a three-dimensional 31P radial Echo-Planar Spectroscopic Imaging sequence (3D radial EPSI) for 31P MRSI in vivo at 7T. The presented data with an isotropic spatial resolution of (10mm)3 in the human calf muscle and (18mm)3 in the human brain show well-resolved loaclized spectra proving feasibility of the 3D 31P radial EPSI sequence with measurement times of about 35min at 7T.
Introduction
Phosphorous magnetic resonance spectroscopy (31P MRS) enables non-invasive observation of high-energy phosphates and metabolites involved in phospholipid turnover. However, 31P MR spectroscopic imaging (31P MRSI) in vivo suffers from low spatial resolution and long measurement times. Combining a readout for echo-planar spectroscopic imaging with a radial k-space sampling scheme could increase the attainable signal-to-noise ratio (SNR) per unit time, thus leading to smaller voxels sizes in measurement times applicable for in vivo applications.
The purpose of this study was to prove feasibility of a three-dimensional 31P radial Echo-Planar Spectroscopic Imaging sequence (3D radial EPSI) for 31P MRSI in vivo at 7T.
Methods
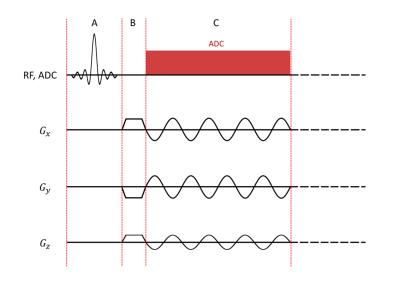
A 3D 31P radial EPSI sequence with sinusoidal readout gradients and continuous sampling of radial k-space trajectories was implemented on a MAGNETOM 7T (Siemens Healthineers, Erlangen, Germany). Projection angles were calculated using an Equal Solid Angle approach1; the sequence further utilizes frequency-selective excitation and signal enhancement by means of the 31P-{1H} nuclear Overhauser effect (NOE). Interleaved acquisition of temporally-shifted gradient echo trains was used to increase the spectral bandwidth.
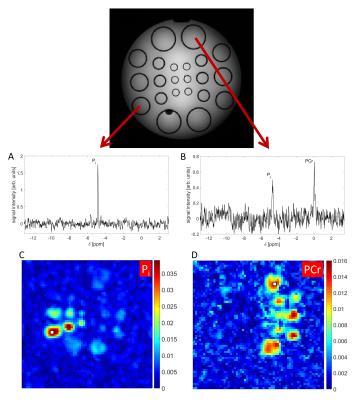
In vitro measurements were performed on a phantom composed of multiple compartments of different diameters ranging from 10mm to 36mm. A Gradient Echo (GRE) image of the phantom is displayed in Figure 1. Half of the compartments contain inorganic phosphate (Pi) in varying concentrations while the other half of the compartments contain the same concentrations of Pi and phosphocreatine (PCr). The gaps between the different compartments are filled with physiologic salt solution (0.9% NaCl). In vivo measurements were performed on the calf muscle and the brain of healthy volunteers (2 male and 1 female, age 24-29y).
All measurements were done using a double-resonant 31P/1H volume coil (Rapid Biomedical, Rimpar, Germany). The 31P radial EPSI datasets were reconstructed using the non-uniform Fast Fourier Transformation algorithm (nuFFT) (MATLAB image reconstruction toolbox of Jeffrey Fessler2). Data correction and post-processing was done using an own MATLAB routine (The MathWorks, Natick, MA, USA). Evaluation of the localized 31P spectra was performed using an own MATLAB implementation of the AMARES algorithm3. 31P spectroscopic images were created by rearranging fitted signal intensities in 3D matrices and registering them with morphological images using MITK4.
Results
The implemented sequence was tested using different acquisition parameter sets (different
echo spacings and resolutions). Up to an isotropic resolution of (8mm)3
and an echo spacing of 0.48ms the threshold for peripheral nerve stimulation
was not exceeded.
Figure 1 shows 3D 31P radial EPSI datasets of the
phantom with an isotropic resolution of (8mm)3 acquired
in 63min. The resonances are well-resolved and no artifacts from gradient
instabilities were observed. The obtained 31P spectroscopic images
show the expected distribution of the metabolites.
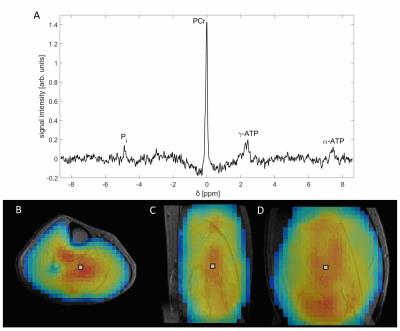
Figure 2 shows 3D 31P-{1H}
radial EPSI datasets with an isotropic
spatial resolution of (10mm)³ acquired from the human calf muscle of a healthy
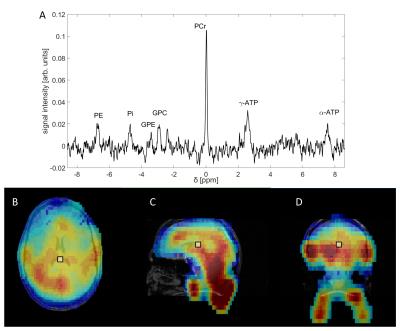
male volunteer in 33min. Figure 3 shows a 3D 31P-{1H} radial EPSI data from the brain of the same
volunteer acquired in 36min with an isotropic spatial resolution of (18mm)³. The
spectra show well resolved resonances of high-energy phosphates and metabolites
involved in phospholipid metabolism. The calculated 31P
spectroscopic images for PCr correspond well with morphological structures in both
cases.Discussion and Conclusion
The results were compared to conventional
Chemical Shift Imaging (CSI) and resulting metabolic maps are consistent
between the different sequences. The sinusoidal shaped readout gradients seem to be a robust and reliable choice for the 31P 3D radial EPSI due to the absence of strong artifacts and spectral quality.
To conclude, the presented data prove feasibility of 3D 31P radial EPSI for 31P MRSI in vivo at 7T with high spatial resolution and measurements times applicable for in vivo studies.
For future applications switching to iterative reconstruction can further
increase the image resolution of spectroscopic images while decreasing
the necessary measurement time, since radial sampled k-spaces are eligible for undersampling.
Acknowledgements
No acknowledgement found.References
1. Lai,
Ching-Ming, and P. C. Lauterbur. "A gradient control device for complete
three-dimensional nuclear magnetic resonance zeugmatographic imaging." Journal
of Physics E: Scientific Instruments 13.7 (1980): 747.
2. Fessler, Jeffrey. "Image reconstruction toolbox." Available at website: http://www.eecs.umich. edu/fessler/code (Accessed September, 2016).
3. Vanhamme, Leentje, Aad van den Boogaart, and Sabine Van Huffel. "Improved method for accurate and efficient quantification of MRS data with use of prior knowledge." Journal of Magnetic Resonance 129.1 (1997): 35-43.
4. Nolden, M. et al. The Medical Imaging Interaction Toolkit: challenges and advances. International Journal of Computer Assisted Radiology and Surgery. 2013 doi: 10.1007/s11548-013-0840-8
Figures