1245
Altered BOLD fluctuations in gliomas and clinical possibilities1Departments of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Fortis Memorial Research Institute, Gurgaon, India, 3Philips India Ltd., Gurgaon, India
Synopsis
In this study, our primary objectives were to characterize the BOLD signal in gliomas using the temporal shift (TS), amplitude of low frequency fluctuations (ALFF) and regional homogeneity (ReHo) measures relative to the contralateral cortex, and to analyse the effectiveness of these measures in distinguishing high grade (HGG) from low grade glioma (LGG). Twenty-one patients with HGG and 13 patients with LGG were investigated. Abnormal hemodynamic fluctuations manifest in HGG, but not for LGG, and can be assessed using functional MRI. BOLD measures reflecting TS and ALFF show promise as an alternative to contrast-enhanced perfusion based techniques in future.
Purpose
Grading of gliomas is an endeavour with many difficulties, but with significant clinical benefit. Despite recent advances in technology the mean survival rate remains dismal, with fewer than 10% of patients with glioblastoma alive after 2 years. One of the reasons for this low rate is the uncertainty in glioma grading. In this study, we evaluated the spontaneous blood oxygen level dependent (BOLD) signal fluctuations to characterize gliomas. Our primary objectives were (i) to characterize the BOLD signal in gliomas using temporal shift (TS)1, amplitude of low frequency fluctuations (ALFF)2 and regional homogeneity (ReHo)3 measures relative to normal appearing contralateral cortex, (ii) to analyse the effectiveness of these measures in distinguishing high grade (HGG) from low grade glioma (LGG) and (iii) to compare these with the Dynamic Contrast-Enhanced (DCE) perfusion derived measures cerebral blood volume (CBV) and time-to-peak (TTP)4.Methods
Twenty-one patients with HGG and 13 patients with LGG were investigated. All patients underwent conventional MRI, DCE-MRI and functional MRI. This study was approved by the institutional ethics committee. Glioma grades were determined by post-surgical biopsy and histology.
BOLD measures such as ReHo, ALFF and TS were determined relative to the cortex of the contralateral hemisphere. CBV and TTP maps were contemporarily evaluated for comparison.
The gliomas were regionally divided into two parts, the core and the outer 4-mm rim, to study the possible spatial variation of the BOLD measures. Also, the 4-mm zone just outside the gliomas (proximith) was analysed. The normal appearing contralateral cortex was used for comparison by normalization of the BOLD and DCE perfusion measures. Student’s t-test was used for statistical assessment of any differences (i) between glioma regions and the entire cortex of the contralateral hemisphere and (ii) between the two glioma grades. Statistical significance was inferred when p<0.05.
Results
We found that the temporal shift was significantly advanced in HGG in comparison to the contralateral cortex, but not for LGG. ALFF and DCE perfusion based CBV maps were significantly higher in HGG than the contralateral cortex and LGG. ReHo and TTP did not show any significant differences between HGG and LGG (table 1).
The temporal shift in HGG was significantly advanced in both HGG regions, the core and rim region, but not proximith. The ALFF of the HGG was significantly higher in the core, but not for the rim, region compared to the contralateral cortex. Outside the tumor region no aberrant effects of the ALFF were found (table 2). For LGG, the ALFF was more or less uniform across core, rim and proximal regions.
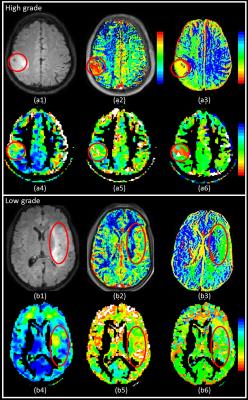
Figure 1 shows native FLAIR images and various parametric maps including CBV, TTP, ALFF, ReHo and TS superimposed in colour on the FLAIR image of a HGG and LGG. The glioma region in HGG was visually different from the surrounding tissue and contralateral cortex using CBV and TS maps, whereas was not in case for LGG.
Discussion and conclusion
Temporal shift maps showed that the hemodynamics were significantly advanced in HGG in comparison to both normal appearing contralateral cortex and LGG. In HGG, it is assumed that, due to the increased tumor vascularization, the microvascular resistance is lower than for the normal contralateral cortex, which might have advanced the hemodynamics in HGG. This interpretation means that the observed temporal shift is a vascular (non-neuronal) effect.
We found that ALFF in HGG were significantly higher than in contralateral cortex and LGG. This is also most likely due to differences in vascularization, blood flow and perfusion between the different grades of gliomas and normal brain tissue. It has been shown with PET studies that the metabolic demand of HGG is higher than for normal appearing tissue, which explains the elevated ALFF in HGG5.
ReHo did not show any significant differences between high and low grade glioma. This could be due to a partial volume-averaging (T2* blurring) effect at the millimetre scale among adjacent heterogeneous areas inside HGG.
In sum, abnormal hemodynamic fluctuations manifest in HGG, but not for LGG, and can be assessed using functional MRI. It appears that quantification of temporal hemodynamic fluctuations may have potential for characterization and clinical grading of gliomas. BOLD measures reflecting the relative temporal shift and ALFF show promise as an alternative to contrast-enhanced perfusion based techniques in future. Noninvasiveness, simplicity, high stability, wide availability, and lower cost of functional MR imaging are all attractive aspects for routine clinical diagnostic application. A non-invasive approach for glioma grading would be very welcome for patients. The approach would be particularly relevant in patients for whom contrast material is contraindicated.
Acknowledgements
No acknowledgement found.References
1. Amemiya S, Kunimatsu A, Saito N, et al. Cerebral Hemodynamic Impairment: Assessment with Resting-State Functional MR Imaging. Radiology 2014;270:548-555.
2. Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83-91.
3. Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. NeuroImage 2004;22:394-400.
4. Sahoo P, Rathore RKS, Awasthi R, et al. Subcompartmentalization of extracellular extravascular space (EES) into permeability and leaky space with local arterial input function (AIF) results in improved discrimination between high- and low-grade glioma using dynamic contrast-enhanced (DCE) MRI. Journal of Magnetic Resonance Imaging 2013;38:677-688.
5. Pauleit D, Stoffels G, Bachofner A, et al. Comparison of (18)F-FET and (18)F-FDG PET in brain tumors. Nucl Med Biol. 2009;36:779-787.
Figures