1244
Silent Sentence Completion Paradigm Shows Superiority in Localization of Wernicke’s Area and Changes in Functional Activation in the Distinct Language Paradigms Correlate with Key Genomic Markers: A Prospective Study1Diagnostic Radiology, MD Anderson Cancer Center, Houston, TX, United States, 2Radiology, Temple University, Philadelphia, PA, United States, 3Neurosurgery, Baylor College of Medicine, Houston, TX, United States, 4Biostatistics, MD Anderson Cancer Center, Houston, TX, United States, 5Neurosurgery, MD Anderson Cancer Center, Houston, TX, United States, 6Neuro-Oncology, MD Anderson Cancer Center, Houston, TX, United States, 7Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States, 8Cancer Systems Imaging, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
The reliability of fMRI for preoperative mapping of language areas depends on the paradigms used, as different tasks harness distinct capabilities to activate areas of speech processing. By comparing 3 language tasks [Silent Sentence Completion (SSC), Category Naming (CAT) and Word Generation (FAS)], we seek to determine the most robust and consistent task in localizing Wernicke’s area. Further the association between genomic markers and functional activation was determined. We included 15 healthy volunteers and 35 patients with gliomas. Results demonstrated that SSC is superior compared to other language paradigms and a correlation exists between tumor genomics and functional activation signals.
PURPOSE
Task fMRI is used for pre-operative localization of eloquent areas in the brain, in order to minimize the risk of post-operative loss of function1,2 . However, specific challenges arrises when mapping receptive speech areas, due to variation in anatomical locations3 and because speech processing has been postulated to be a function of intertwined neuronal mechanisms that involve complex higher brain functions4,5. Thus, commonly used paradigms have shown inconsistency when mapping Wernicke’s area4,6,7. At its core, our study aims to determine the adequacy and robustness of silent sentence completion (SSC) in localizing Wernicke’s area compared to Category Naming (CAT) and Word Generation (FAS). Furthermore, we propose an association between functional activation patterns and signal intensity with the following key genomic markers: tumor protein 53 (TP53), phosphatase and tensin homolog (PTEN), epidermal growth factor receptor (EGFR), isocitrate dehydrogenase 1 and 2 (IDH1/2), 1p/19q co-deletion, S-100, and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation.MATERIALS AND METHODS
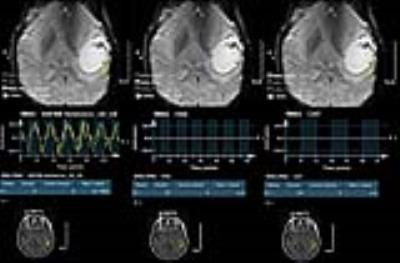
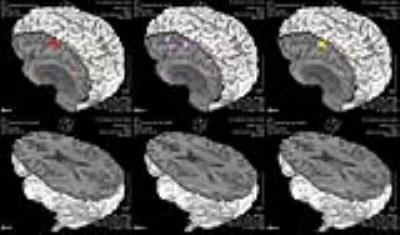
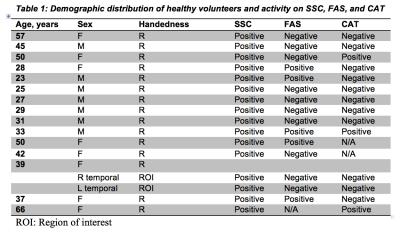
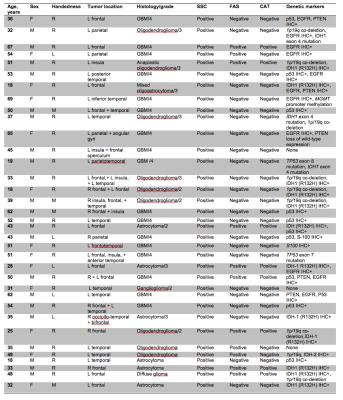
This IRB approved study included 15 right-handed healthy controls and 35 mixed handed brain tumor patients referred for pre-surgical mapping (Tables 1, 2). The inclusion criteria for cancer patients were: 18 to 75 years of age, no contraindications to MRI, evidence of brain mass on imaging, fMRI examination for brain mapping. Patients’ ability to perform tasks accurately was based on the neuropsychology team’s overall assessment of their cognitive profile and behavioral observations. Three language tasks were administered: SSC, CAT and FAS. All paradigms were block designed, with alternating active and control blocks. Each paradigm had a duration of 4 min and 20 seconds, with blocks alternating for 20 seconds each. All tasks were designed for clinical purpose and were presented visually. Imaging data included a high-resolution T1 weighted structural image (TR/TE=, 1x1x1mm3) and a series of BOLD EPI scans (TR/TE= ms, 4x4x4mm3). Data analysis was performed using Dynasuite Neuro 3.0 workstation (In Vivo Corporation, Gainesville, FL, USA). ROI localization was performed systematically; an experienced neuroradiologist (R.C.C., with 7 years of clinical experience with fMRI) mainly focused on the temporal lobe, with a more narrowed focus on the areas of the posterior superior temporal, middle temporal, and angular gyri, which correlate with the anatomical location of Wernicke’s area. Potential ROIs corresponding to Wernicke’s activation were assessed on all 3 tasks for the presence or absence of activity. A statistical threshold of the activation map was used to maximize signal to noise ratio, with a P ≤ 0.05 (bonferroni corrected). The detection rates of SSC, FAS, and CAT were summarized using frequencies and percentages. McNemar’s test was used to compare the detection rate between paradigms. Fisher’s exact test was used to assess the association between detection status and genomic marker status for FAS and CAT. Wilcoxon rank sum test was used to assess the association between signal intensity and genomic marker status for SSC.
Signal Intensity = (Number of voxels active)/(Total Number of voxels in ROI)
RESULTS
In healthy volunteers SSC showed 100% activation of Wernicke’s area (Table 1) whereas FAS and CAT detected significantly fewer cases (p=0.004 and 0.003 by McNemar’s test, respectively). SSC had a 100% positive detection rate in cancer patients (N=35) (Table 2), whereas FAS had a positive rate of 28% (10 of 36 ROI’s), and CAT had a rate of 22% (8 of 36 ROI’s) . No significant difference was detected between FAS and CAT (P-value=0.08) (Fig. 1&2).
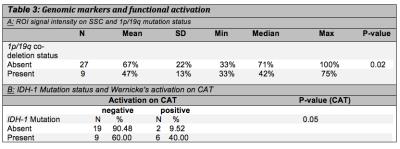
With regard to rates of functional activation, Patients with IDH1 wild-type were more likely to be classified as negative on CAT; a higher percentage of patients with IDH1 wild-type tumors showed no activation (90.48% versus 9.52%) versus those with IDH1 mutation (60% versus 40%) (p-value = 0.05) (Table 3a).
Relating to signal intensity patients without 1p/19q co-deletion showed a higher median magnitude of signal intensity compared with 1p/19q co-deletion (71% versus 42%; p-value=0.02) (Table 3b).
DISCUSSION/CONCLUSIONS
As demonstrated from our results, SSC is superior to FAS and CAT at localizing Wernicke’s area. SSC showed high activation rates in Wernicke’s area across various types of gliomas and anatomical locations, reflecting its efficacy and consistency. Our analysis further elucidated statistically significant associations between certain key genomic markers and functional activation, suggesting that tumoral genomic profile, if known, might eventually dictate the use of a specific battery of language tasks. Future research will have to validate these findings by comparing them with the gold standard intraoperative functional mapping and postsurgical clinical outcomes.Acknowledgements
This research was conducted at the MD Anderson Center for Advanced Biomedical Imaging, with equipment support from General Electric Healthcare and partially funding from the Diagnostic Imaging Clinical Research Committee.
John S. Dunn Sr. Distinguished Chair in Diagnostic Imaging Fund
MD Anderson Cancer Center startup funding
MD Anderson Brain Tumor Center Program
References
1. Colen, R.R., H. Kekhia, and F.A. Jolesz, Multimodality intraoperative MRI for brain tumor surgery. Expert Rev Neurother, 2010. 10(10): p. 1545-58.
2. Ogawa, S., et al., Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A, 1990. 87(24): p. 9868-72.
3. Faro, S.H. and F.B. Mohamed, Functional MRI basic principles and clinical applications. 2006, Springer: New York, NY. p. 1 online resource (xiii, 533 p.).
4. Zaca, D., S. Jarso, and J.J. Pillai, Role of semantic paradigms for optimization of language mapping in clinical FMRI studies. AJNR Am J Neuroradiol, 2013. 34(10): p. 1966-71.
5. DeWitt, I. and J.P. Rauschecker, Wernicke's area revisited: parallel streams and word processing. Brain Lang, 2013. 127(2): p. 181-91.
6. FitzGerald, D.B., et al., Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol, 1997. 18(8): p. 1529-39.
7. Lurito, J.T., et al., Comparison of fMRI and intraoperative direct cortical stimulation in localization of receptive language areas. J Comput Assist Tomogr, 2000. 24(1): p. 99-105.
Figures