1239
Functional connectivity of DLPFC circuits predicts cocaine relapse1Neuroimaging Research Branch, Intramural Research Program, National Institute on Drug Abuse, Baltimore, MD, United States
Synopsis
TMS targeting the DLPFC has been shown to effectively manipulate inter-temporal behaviors in healthy individuals and drug-using behaviors in cocaine users. However, the neural mechanism through which the DLPFC is involved in the alteration of these behaviors remains unclear. In the current study, we utilized resting-state fMRI to investigate the relationship between the DLPFC functional connectivity and relapse in cocaine addiction. Our voxel-wise Cox regression analyses revealed that two DLPFC circuits have protective effects against cocaine relapse.
Introduction
Neurobiological and neuroimaging studies have demonstrated that the dorsal lateral prefrontal cortex (DLPFC), a major component of the executive control network, is crucial in modulating top-down control and regulating decision-making 1,2. Transcranial magnetic stimulation (TMS) targeting the DLPFC has been shown to effectively manipulate inter-temporal behaviors in healthy individuals 3 and drug-using behaviors in cocaine users 4. However, the neural mechanism through which the DLPFC is involved in the alteration of these behaviors remains unclear. In this study, we utilized resting-state fMRI (rs-fMRI) to investigate the relationship between the DLPFC functional connectivity (FC) and relapse in cocaine addiction.Methods
Forty-five cocaine use disorder (CUD) patients and 22 healthy control (HC) subjects participated in this study. The CUD participants underwent one of three residential cocaine-dependence treatment programs that utilized the Minnesota Model psychosocial treatment approach. Urine drug screens were conducted throughout the residential treatment to verify abstinence. The CUD participants were followed for 168 days post-treatment or until relapse to stimulant use. The study was approved by the Institutional Review Boards and written informed consent of each subject was obtained prior to the study 5. Resting-state fMRI data were obtained on a Philips 3T scanner using a GE-EPI sequence (TE/TR=25/1700 ms, 212 volumes). The rs-fMRI datasets were analyzed with AFNI, SPM8 and Matlab. Slice timing correction, head motion correction, white matter, CSF signal removal were performed. The necessity of global signal removal was determined individually by evaluating global negative index of each subject 6. Band-pass filter was used to keep low-frequency fluctuation between 0.012-0.1Hz. The datasets were then normalized to standard MNI space and resampled to isotropical 2mm. The seed ROI located on the left DLPFC was selected in accordance with literature 4. The cross-correlation coefficient map of each subject was generated by correlating time course of each voxel with that of the seed. After applying Fisher’s Z-transformation, the datasets of HC subjects were used to generate a pattern with significant FC to the seed which served as a mask for following analyses. Then we utilized a customized Matlab program to conduct relapse prediction based on the Cox regression model, in a voxel-wise fashion, to generate the relative hazard ratio (HR) map. Age, gender, years of education and mean head movement during the scan were served as covariates in the Cox regression analysis. To further evaluate the potential ability of the Cox model in predicting relapse at an individual level, we also performed the leave-one-out (LOO) cross validation. All analyses were thresholded at the rigorous p=0.001 and Monte-Carlo simulation implemented with 3dclustsim program of AFNI was used to correct for multiple comparisons. To evaluate the prediction power of the above analyses, we conducted receiver operating characteristic (ROC) analyses and calculated the area under curve (AUC) of the ROC of each voxel to generate the AUC maps.Results
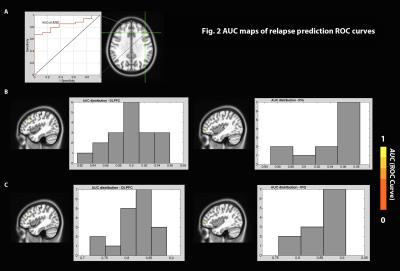
The voxel-wise Cox regression analysis revealed that two regions in the left DLPFC and left inferior frontal gyrus (IFG) survived the statistical threshold and both had HR values < 1 (Fig.1). Individuals with higher connectivity between these regions and the seed had lower relative relapse HR (or longer abstinence following the treatment), suggesting protective effects of the connectivity of these brain circuits against relapse. Fig. 2 shows the result of the ROC analyses for both without and with the LOO analyses of these two regions. AUCs of the ROC curves are ranging from 0.82~0.96 for the left DLPFC (Fig.2B left), and from 0.85~0.99 for the left IFG (Fig.2B right) without LOO. With the LOO cross-validation, the AUC ranges dropped to 0.72~0.89 for the left DLPFC (Fig.2C left) and 0.76~0.91 for the left IFG (Fig.2C right).Discussion and Conclusion
Emerging concepts based on neuroimaging studies suggest that cognitive behaviors were supported by neural networks connected among discrete brain regions 7. The observed regions in the current study (the DLPFC and IFG) that serve as a protective factor against relapse are in accordance with principle of TMS with long-term potentiation effects that stimulating the target could lead to a momentary elevation of the BOLD signal in the directly stimulated area, as well as regions monosynaptically connected to the stimulated site 8,9. These findings may serve as the neural mechanism of the effective manipulation of the behavioral outcome by TMS targeting the DLPFC, and extend our understanding of the neural underpinnings of the high relapse in addiction. The approach used in the study may have the potential to guide informed treatment such as evaluating the effectiveness of the stimulus site in TMS treatment.Acknowledgements
The research was supported by the Intramural Research Program of the NIH, NIDA. We thank Dr. Bryon Adinoff for providing data on cocaine users.References
1. Hare et al. Self-control in decision-making involves modulation of the vmPFC valuation system, Science. 2009;324(5927): 646-648.
2. Baumgartner et al. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice, Nature neuroscience. 2011;14(11): 1468-1474.
3. Figner et al. Lateral prefrontal cortex and self-control in intertemporal choice, Nature neuroscience. 2010;13(5): 538-539.
4. Terraneo et al. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study, European Neuropsychopharmacology. 2016;26(1): 37-44.
5. Adinoff et al. Basal hippocampal acivity and its functional connectivity predicts cocaine relapse, Biological Psychiatry. 2015;78(7): 496-504.
6. Chen et al. A Method to Determine the Necessity for Global Signal Regression in Resting-State fMRI Studies, Magnetic Resonance in Medicine. 2012;68(6): 1828-1835.
7. Mesulam, M. Defining neurocognitive networks in the BOLD new world of computed connectivity, Neuron. 2009;62(1): 1-3.
8. Hanlon et al. Mobilization of Medial and Lateral Frontal-Striatal Circuits in Cocaine Users and Controls: An Interleaved TMS/BOLD Functional Connectivity Study, Neuropsychopharmacology. 2016 (Epub ahead of print).
9. Bestmann et al. On the synchronization of transcranial magnetic stimulation and functional echo-planar imaging, Journal of Magnetic Resonance Imaging. 2003;17(3): 309-316.
Figures
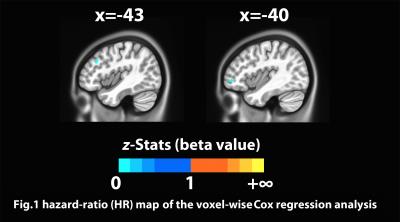
Fig.1 hazard-ratio (HR) map of the voxel-wise Cox regression analsis using the left DLPFC functional connectivity, HR value > 1 indicate region(s) with risk effect to relapse, HR value between 0 and 1 indicate region(s) with protective effect against relapse.
(voxel-wise thresholded at p=0.001, family-wise corrected with α=0.05, minimum cluster size = 96mm3)