1238
Top-down modulation in the visual cortex negatively correlates with duration of blindness and reaction time during sensory substitution1Neuroimaging Laboratory, University of Pittsburgh, Pittsburgh, PA, United States, 2Department of Ophthalmology, University of Pittsburgh, Pittsburgh, PA, United States, 3Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
Visual cortex functionality in the blind has been shown to shift away from sensory networks toward task-positive networks that are involved in top-down modulation. However, how such modulation is shaped by experience and reflected behaviorally remains unclear. Using blood-oxygenation-level-dependent functional MRI with a sensory substitution task, we found that top-down visual cortex activity negatively correlates with duration of blindness and reaction time. Our results suggest that alterations in top-down brain activity due to visual deprivation progress as a function of time. Furthermore, the degree of top-down activity in the visual cortex may reflect the speed of performance during sensory substitution.
Purpose
Visual cortex functionality in the blind has been shown to shift away from sensory networks toward task-positive networks that are involved in top-down modulation1. However, how such modulation is shaped by experience and reflected behaviorally remains unclear. This study evaluated the top-down visual cortex activity as a function of blindness duration or behavioral response using BOLD functional MRI with a sensory substitution task.Methods
Four congenitally blind subjects (age= 50.8±17.5 years), 7 acquired blind subjects (age= 45.4±16.6 years) and 11 sighted controls (age= 49.9±16.2 years) were examined (mean ± standard deviation, p= 0.82 for ANOVA on age) using a 3 Tesla Siemens Allegra MRI scanner after obtaining informed written consent. These subjects were naïve to the auditory sensory substitution device known as the vOICe2, and were presented a series of soundscapes that represented bars moving across the image in one of four directions. In order to examine top-down influence on the visual cortex during sensory substitution, each subject underwent two block-design BOLD functional MRI scans with identical sound stimuli within the same experimental session in the scanner. Subjects first passively listened to soundscapes without any prior knowledge of the vOICe (Pre-training). Immediately after the pre-training scan, subjects were instructed on how images are encoded as soundscapes and asked to actively interpret the sounds as images during the second scan (Post-training). They also responded with a keypad placed in their right hand as soon as they could discern the direction of motion. Sighted subjects wore a blindfold for all scans and training.
BOLD functional MR images were collected with a single-shot gradient-echo echo-planar-imaging pulse sequence. The block design consisted of 15 trials with alternating 14 s of rest and 10 s of task. BOLD activation maps were calculated using a general linear model. The average BOLD% change was calculated in 3 regions of interest (ROIs) corresponding to the primary, secondary and tertiary visual cortices. To determine the degree of top-down modulation in the visual cortex, we subtracted the pre-training BOLD% change from the post-training BOLD% change (∆BOLD%) for each of the 3 ROIs in each subject. The relationships between ∆BOLD% and duration of blindness or behavioral reaction time were then tested by non-parametric Spearman correlations.
Results
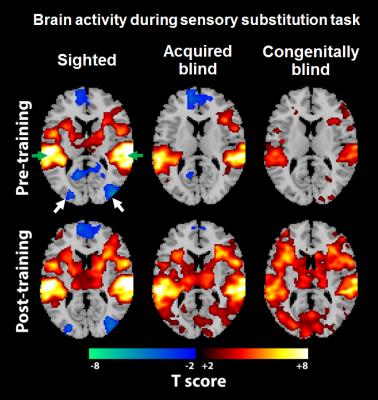
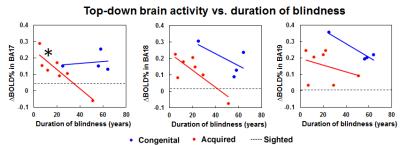
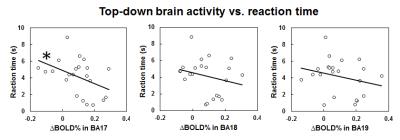
Figure 1 shows the BOLD activation maps of the brains of sighted, acquired blind and congenitally blind subjects in response to a visual-to-auditory sensory substitution task before and after 10 min of training in the MRI scanner. While our sensory substitution task showed similar levels of negative BOLD response in the occipital visual cortex of sighted subjects before and after training, both acquired blind and congenitally blind subjects showed increased positive BOLD responses in the visual cortex after training, indicating increased top-down modulation of the visual cortex in the blind but not sighted subjects. Interestingly, when comparing the degree of top-down modulation in the visual cortex across duration of blindness, our further analyses showed a decreasing BOLD activity change in the primary visual cortex of acquired blindness with increasing blindness duration (Figure 2). The degree of top-down modulation in primary visual cortex also negatively correlated with reaction time during sensory substitution (Figure 3).Discussion
In blind individuals, the visual cortex may respond to non-visual stimuli such as sound localization3, auditory movement4 and detection of sound changes5. However, the role of the visual cortex in interpreting such stimuli remains unclear6. The amplitude and size of cortical activation in BOLD functional MRI have been shown to negatively correlate with reaction time in sighted subjects during visual tasks7,8. Here, we demonstrated that stronger top-down activity in the visual cortex may reflect faster responses during auditory sensory substitution. Our results showing decreasing top-down activity in the visual cortex of subjects with acquired blindness over time also indicated that the plasticity of the visual system can gradually be reshaped after late onset of blindness beyond the sensitive period9,10.Conclusions
This work represents an early step toward understanding plasticity in the visual system and how it depends upon prior experience. Our results suggest that alterations in top-down brain activity due to visual deprivation progress as a function of time. Furthermore, the degree of top-down activity in the visual cortex may influence the speed of performance during sensory substitution tasks. In the long term, discovering biomarkers of visual plasticity could aid in the screening of patients who are candidates for vision restoration devices. Longitudinal measures of brain activity may be essential for characterizing the dynamic states of the visual system.Acknowledgements
This work was supported by the National Institutes of Health Contracts P30-EY008098 and T32-EY017271-06 (Bethesda, Maryland); United States Department of Defense DM090217 (Arlington, Virginia); Alcon Research Institute Young Investigator Grant (Fort Worth, Texas); Eye and Ear Foundation (Pittsburgh, Pennsylvania); Research to Prevent Blindness (New York, New York); Aging Institute Pilot Seed Grant, University of Pittsburgh (Pittsburgh, Pennsylvania); and Postdoctoral Fellowship Program in Ocular Tissue Engineering and Regenerative Ophthalmology, Louis J. Fox Center for Vision Restoration, University of Pittsburgh and UPMC (Pittsburgh, Pennsylvania).References
[1] Murphy MC, Nau AC, Fisher C, Kim SG, Schuman JS, Chan KC. Top-down influence on the visual cortex of the blind during sensory substitution. Neuroimage. 2016;125:932-40. [2] Meijer PB. An experimental system for auditory image representations. IEEE transactions on bio-medical engineering. 1992;39(2):112-21. [3] Lessard N, Pare M, Lepore F, Lassonde M. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395(6699):278-80. [4] Poirier C, Collignon O, Scheiber C, Renier L, Vanlierde A, Tranduy D, et al. Auditory motion perception activates visual motion areas in early blind subjects. Neuroimage. 2006;31(1):279-85. [5] Kujala T, Palva MJ, Salonen O, Alku P, Huotilainen M, Jarvinen A, et al. The role of blind humans' visual cortex in auditory change detection. Neurosci Lett. 2005;379(2):127-31. [6] Collignon O, Lassonde M, Lepore F, Bastien D, Veraart C. Functional cerebral reorganization for auditory spatial processing and auditory substitution of vision in early blind subjects. Cerebral cortex. 2007;17(2):457-65. [7] Verghese A, Kolbe SC, Anderson AJ, Egan GF, Vidyasagar TR. Functional size of human visual area V1: a neural correlate of top-down attention. Neuroimage. 2014;93 Pt 1:47-52. [8] Mohamed MA, Yousem DM, Tekes A, Browner N, Calhoun VD. Correlation between the amplitude of cortical activation and reaction time: a functional MRI study. AJR Am J Roentgenol. 2004;183(3):759-65. [9] Voss P. Sensitive and critical periods in visual sensory deprivation. Front Psychol. 2013;4:664. [10] Cohen LG, Weeks RA, Sadato N, Celnik P, Ishii K, Hallett M. Period of susceptibility for cross-modal plasticity in the blind. Ann Neurol. 1999;45(4):451-60.Figures