1234
Measurement of placental oxygenation in a guinea pig model of intrauterine growth restriction1Medical Biophysics, University of Western Ontario, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada, 3Obstetrics and Gynaecology, University of Western Ontario, London, ON, Canada, 4Division of Maternal, Fetal and Newborn Health, Children's Health Research Institute, London, ON, Canada, 5Physiology and Pharmacology, University of Western Ontario, London, ON, Canada
Synopsis
We sought to examine the placental oxygenation status in a guinea pig model of intrauterine growth restriction (IUGR). We measured T2* in placentae of IUGR and control fetuses during a maternal oxygen challenge, where imaging was performed at both 20% and 100% inhaled oxygen. IUGR was defined by an elevated brain to liver volume ratio, indicative of blood flow redistribution secondary to fetal hypoxia. No significant difference in ΔT2* was observed, indicating that the placentae of the IUGR fetuses were not hypoxic. Thus we concluded that placental hypoxia is not necessary to induce fetal hypoxia in the guinea pig.
Introduction
Intrauterine growth restriction (IUGR) affects up to 10% of all pregnancies and is a leading cause of perinatal morbidity and mortality.1 IUGR resulting from placental insufficiency is often associated with fetal hypoxia.2 Oxygen supply through the placenta is the major driver for fetal growth, however, fetal hypoxia is not always associated with placental hypoxia.2
In the current study, we sought to examine the placental oxygenation status in a guinea pig model of IUGR, in which an increased brain to liver volume ratio (BLVR) indicates redistribution of fetal blood flow in response to fetal hypoxia.3 We measured T2* during a maternal oxygen challenge in IUGR and control placentae, where imaging was performed at both 20% and 100% inhaled oxygen, as previously reported.4 T2* differences observed during a maternal oxygen challenge are indicative of oxygen saturation (SO2) and therefore provide important information about placental oxygenation status.4
Methods
To induce IUGR, vessel occluders were placed bilaterally on the uterine arteries at 35 days gestation (term ~68 days, N = 3, 11 fetuses).5 Sham surgeries were also performed to produce a control group (N = 2, 11 fetuses). At ~60 days gestational age, sows were anaesthetized using isoflurane (1.5-2.5%) and scanned at 3T (MR750, GE, Waukesha, WI) using a 32 channel receive coil under a protocol approved by the institution’s Animal Care Subcommittee. Anatomical T1- and T2-weighted images were acquired for visualization and segmentation purposes. A gradient echo T2* mapping sequence (TR/TE1/ΔTE/# of echoes = 2000ms/1396ms/6072ms/16) was performed with the maternal guinea pig breathing air with an oxygen concentration of 20%. The animal was then switched to 100% oxygen and the imaging was repeated after 10 minutes. T1- and T2-weighted images were used to segment the fetal liver, brain, and placentae, which were matched to the corresponding fetus. T2* maps were calculated by fitting the multi-echo images to an exponential decay function in Matlab (Mathworks, Natick, MA). Placental ROIs from the T1-weighted images were copied onto the T2* maps to obtain values for each placenta. T2* values were also calculated in the maternal kidneys to ensure validity of the technique.Results
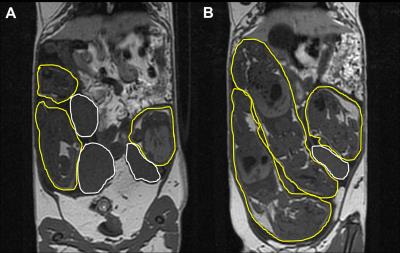
For this work, fetuses in the surgical group were defined as IUGR if they had a BLVR >0.7. This cutoff was chosen based on the normal distribution of BLVR in sham surgery animals, updated from previous work by our group.6 One spontaneously growth restricted control fetus was removed from the analysis. Thus our study population consisted of 5 IUGR fetuses and 10 control fetuses. Figure 1 shows T1- and T2-weighted images, highlighting the fetuses and the placentae, of a pregnant guinea pig that underwent bilateral uterine artery occlusion surgery. T2* values in the maternal kidneys increased in response to the increased concentration of inhaled oxygen in all cases (ΔT2* = 4.3 ± 4.2ms, range = 0.01 – 12.23ms). In the placentae, a ΔT2* difference was not observed between sham control and IUGR fetuses (P > .05; Figure 2A). Further, no correlation was observed between ΔT2* and BLVR (R2 = .13; Figure 2B).Discussion
In this study, we observed no difference in ΔT2* between control and IUGR fetuses. Furthermore, the ΔT2* values for the IUGR group were not different from zero, even though the maternal kidneys responded to the increased oxygen availability. Taken together, these results suggest that the placentae of the IUGR fetuses were not hypoxic, indicating that oxygen delivery to the placenta had not been compromised. Rather, as elevated BLVR is associated with a hypoxic fetus,3 this suggests an impairment of oxygen diffusion from placenta to fetus.
Our study supports the concept of ‘postplacental hypoxia’ that has been suggested based on measurements of uterine and umbilical venous PO2 in animal models of IUGR and of molecular markers of oxygen status in human placentae.2 In future studies, these oxygenation measurements in the fetal liver and brain could give further insight into the delivery of oxygen from the placenta to the hypoxic fetus.
Conclusion
We have demonstrated in a guinea pig model of IUGR that placental hypoxia is not necessary to induce hypoxia in the fetus after bilateral uterine artery occlusion.Acknowledgements
Research reported in this abstract was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (U01HD087181), the Natural Sciences and Engineering Research Council (RGPIN-356310-2013) and the Canada Research Chairs Program (950-228038).References
1. Vandenbosche RC, and Kirchner JT. Intrauterine growth retardation. Am Fam Physician. 1998;58(6):1384-90.
2. Kingdom JC, and Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613-21.
3. Visentin S, Grumolato F, Nardelli GG, et al. Early origins of adult disease: Low birth weight and vascular remodeling. Atherosclerosis. 2014;237(2):391-99.
4. Chalouhi GE, Alison M, Deloison B, et al. Fetoplacental oxygenation in an intrauterine growth restriction rat model by using blood oxygen level-dependent MR imaging at 4.7 T. Radiology. 2013;269(1):122-9.
5. Herrera EA, Alegria R, Farias M, et al. Assessment of in vivo fetal growth and placental vascular function in a novel intrauterine growth restriction model of progressive uterine artery occlusion in guinea pigs. J Physiol. 2016;594.6:1553-1561.
6. Sinclair KJ, Friesen-Waldner LJ, McCurdy CM, et al. Examining intrauterine growth restriction due to placental insufficiency in fetal guinea pigs in utero using MRI [Abstract]. Proceedings of the 23rd Annual meeting of ISMRM; 2015. Abstract no. 4013.
Figures