1224
Sub-Millimeter Cortical Imaging at 7T using a High-Density Motor-Cortex 32-Channel Array Coil1Institute of Medical Physics and Radiation Protection, Department of Life Science Engineering, Mittelhessen University of Applied Sciences (THM), Giessen, Germany, 2A.A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 3Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States, 4Helen Wills Neuroscience Institute, UC Berkeley, Berkeley, MA, United States, 5Advanced MRI Technologies, Sebastopol, CA, United States
Synopsis
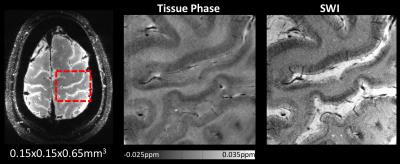
A densely packed 32-channel motor cortex array coil was designed, constructed and compared to a 32-channel whole-head coil at 7T. The developed design allows coil adaptability to a wide range of head sizes, thereby minimizing the distance of the brain to the individual small loop elements. Using the high SNR and parallelism afforded by this array, a substantial gain in sensitivity and performance for imaging the human motor cortex was achieved, enabling 0.15x0.15x0.65 mm3 resolution SWI scans.
Introduction
32-channel brain arrays have become increasingly
used for both clinical and research applications at 7T. Accelerated parallel
imaging and ultra-high magnetic field are technologies that are synergistic and
complementary. High fields are expected to improve parallel imaging performance
due to the more distinguishable sensitivity profiles of each coil element1. Motor
cortex imaging presents a particular opportunity for high acceleration rates
with good SNR, since the bulk of the motor cortex is close the skull and it is
located bilaterally on the sides of the brain and head. Here, we tested whether
a local array coil, closely fitting to the motor cortex and with dense packing
of 32 channels, would result in sufficient SNR gain to realize highly
accelerated sub-millimeter imaging at 7T. Methods
A critical component of our development was the identification and implementation of the coil former shape in order to find an optimized coil topology for motor cortex imaging. The array comprises of 32 small coil elements (diam: 38mm) where sample noise dominance across different head sizes becomes a critical factor. We implemented a split “horse-shoe” topology (Fig 1), where the two housing segments are hinged together. This design allows coil adaptability to a wide range of head sizes, while maintaining strong coil-to-sample coupling. Each lateral coil segment comprises 16 circular elements, which are laid out using hexagonal tilling pattern2. Nearest neighbor decoupling was achieved by overlap (avg. -14dB), with further and next-neighbor decoupling achieved by the preamp decoupling2 (-17dB). Preamplifiers are connected directly to the elements via a customized daughter board. Thus, no connecting cable is needed. This allows the coil’s drive port to be included on the front-end of the preamp’s daughter board. The latter comprises a capacitance voltage divider and a series matching capacitor. The same circuitry has both active and passive detuning trap (crossed passive diode) incorporated. A separately housed 16-rung CP birdcage coil with an inner diameter of 30 cm was used for transmission. Data were acquired on a 7T scanner (Magnetom, Siemens Healthcare) and compared to a custom built 32-channel whole-head coil3. SNR and g-factor maps were obtained from 3mm isotropic resolution PD-weighted FLASH images (TR/TE/α:300ms/15ms/10°, matrix:256x256). Since the 32-channel whole-head coil and motor cortex coil uses different transmit coils, spatially varying flip angle across the head and the Tx coils were taken into account by incorporating the B1+ map into the SNR calculations. The array was tested in-vivo with a high-resolution anatomical SWI acquisition (TR/TE/α:450ms/23.7ms/40°, matrix:1066x924, with cardiac gating, flow compensation, 2 averages, and full k-space sampling).Results
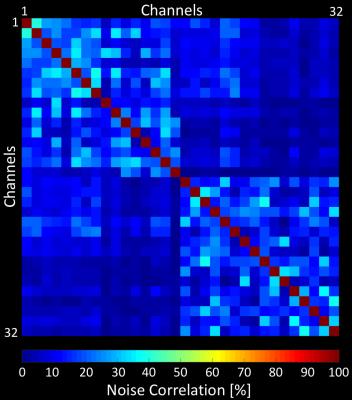
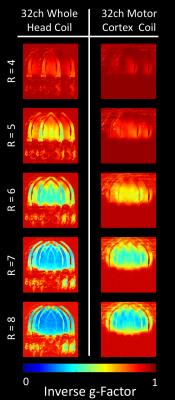
The QU/QL-ratio of the 38 mm loop was measured to be 3.1. The array’s noise correlation ranges (Fig. 2) from 0.1% to 41% (avg.: 8.2%). The developed 32-channel motor cortex coil exhibited a 2-fold SNR improvement compared to a 7T whole-head 32-channel coil at the targeted cortical brain region (Fig 3). At a distance of 4cm from the edge of the brain the SNR was equal to SNR obtained from the 32-channel head coil. Central brain SNR was decreased by 8%. In addition, the small loop sizes provided an increased diffraction limit in parallel imaging encoding, empowering acceleration at 1.5 factors higher with similar noise amplification compared to the 32-channel whole-head coil (Fig 4). Volunteer tests have shown that ultra-high resolution images were feasible, enabling 0.15x0.15x0.65 mm3 resolution SWI scan with acquisition time of ~20 min. (Fig. 5).Discussion
We were able to overcome a number of technical challenges to designing and constructing a dense array receive coil and demonstrated the capability of such a coil for ultra-high resolution anatomical imaging of the human motor cortex. Array coils with small elements are technically challenging because the inter-element decoupling becomes more difficult and time-consuming as the element density increases. Maintaining a high QU/QL-ratio for such small loops is a challenge that we overcame by using a semi-adaptable coil former, thereby minimizing the distance of the brain to the individual elements. The high SNR and parallelism allowed us to image at substantially higher resolution compared to a 32-channel whole-head coil. A larger high-density array to cover the entire head would require many more receiver channels (e.g. 128ch), but it could extend the obtained SNR gain across the whole cerebral cortex, while maintaining central brain SNR.Conclusion
A densely packed 32-channel motor cortex array coil was designed, constructed and compared to a 32-channel whole-head coil at 7T. This new cortex array coil was able to realize substantial gain in SNR and performance for imaging the human motor cortex and surrounding associated sensory motor areas of the brain.Acknowledgements
NIH P41RR14075, NIH BRAIN Initiative R24MH106096References
1. Wiesinger F, Boesiger P, and Pruessmann KP. Electrodynamics and Ultimate SNR in Parallel MR Imaging. Magn Reson Med. 2004;52(2):376-390.
2. Roemer PB, Edelstein WA, Hayes CE, et al. The NMR Phased Array. Magn Reson Med. 1990;16(2):192-225.
3. Keil B, Triantafyllou C, Hamm M, et al. Design Optimization of a 32-Channel Head Coil at 7T. In proc ISMRM. 2010;18:1493.
4. Wei H, Zhang Y, Gibbs E, et al. Joint 2D and 3D phase processing for quantitative susceptibility mapping: application to 2D echo-planar imaging. NMR in Biomed. 2016 (Epub ahead of print).
5. Kaaouana T, de Rochefort L, Samaille T, et al. 2D harmonic filtering of MR phase images in multicenter clinical setting: Toward a magnetic signature of cerebral microbleeds. NeuroImage. 2015;1(104):287-300.
Figures