1222
A 32-channel loop-dipole transceiver array for body imaging at 7.0 Tesla1Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
A 32-channel transmit/receive (transceiver) body array (32LD) was developed by combining eight dipole elements with 24 loops, 3 each stacked lengthwise under each dipole. The transmit/receive performance was compared against a 16-channel loop-dipole (16LD) array in numerical simulations and phantom experiments. The 32LD had improved SNR near the surface and enabled accelerations in foot-head dimension as expected. Inside deeply situated organs, SNR of 32LD was comparable to 16LD. Furthermore, the 32LD had ~30% higher local SAR than the 16LD with single-spoke RF shimming inside the kidneys and torso. High channel count body imaging arrays can be developed by combining dipoles and loops with geometric decoupling.
Purpose
Compared to the use of dipole antenna elements alone [1], combining loops and dipoles have been demonstrated to improve imaging performance inside the body at 7.0T while maintaining good decoupling performance between channels [2]. In this work improving upon a previous 16-channel loop-dipole design [2], we aim to develop a 32-channel transmit/receive (transceiver) body imaging array at 7.0T with improved parallel transmit and parallel receive performance over existing arrays, while utilizing the capabilities of 32-channel parallel transmit hardware for body imaging.Methods
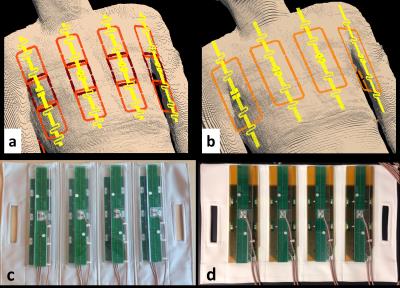
The 32-channel combined loop-dipole transceiver array (32LD) consisted of 8 identical loop-dipole building blocks, with each block containing a fractionated dipole antenna [1] and three 6x9cm2 rectangular loop coil elements. Loop coils were centered around the dipole elements, placed in three rows along the z-dimension and were overlapped by 1cm to minimize mutual coupling between nearest neighbors. The dipole and loop elements were etched on separate FR4 boards, tuned/matched using fixed value capacitors and aligned along their long-axes at their center locations in order to reduce mutual coupling between dipole and loop elements [2]. Elements of the array were placed in two flexible vinyl fabric housings [2], with a center-to-center distance of ~11cm between neighboring blocks (Figure 1).
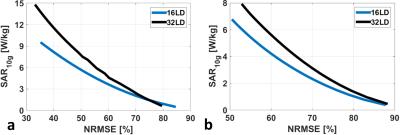
The 32LD was modeled around the chest, torso and pelvis of an anatomically correct human model (Duke, Virtual-Family [3]). EM-field distributions of each coil element were computed using an FDTD solver in SEMCAD X software (SPEAG, Zürich, Switzerland) and were imported to Matlab (Mathworks, Natick, MA) to investigate parallel transmit, receive and parallel imaging performance. Transmit performance was evaluated using SAR-constrained single-spoke magnitude/phase shimming and plotting L-curves (i.e. peak SAR vs normalized root-mean-squared error) [4]. Signal-to-noise ratio (SNR) was quantified in a root sum-of-squares fashion inside the prostate, kidneys, heart and a 15cm-long region of torso covering kidneys (torso). Noise amplification factors (g-factors) were calculated along a coronal slice inside the torso intersecting both kidneys for reductions along left-right (L-R) and foot-head (F-H) dimensions. Transmit and receive performance of the 32LD was compared against a 16-channel loop-dipole transceiver array (16LD) [2] using identical numerical methods.
Experimental data were acquired inside a torso-sized phantom (εr=77.8, σ=0.82S/m) [5] on a Siemens Magnetom 7.0T whole body MRI scanner (Siemens Healthcare, Erlangen, Germany) equipped with 32 independent 1-kW power amplifiers managed through a separate phase and gain controller (Communications Power Corporation, Hauppauge, New York). Flip angle and SNR maps were acquired and calculated using the sequences and methods in Ref. [4].
Results
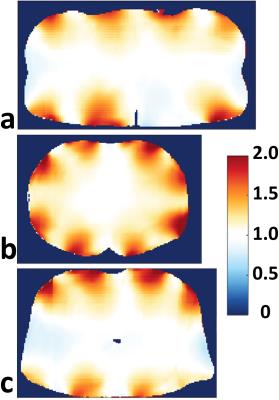
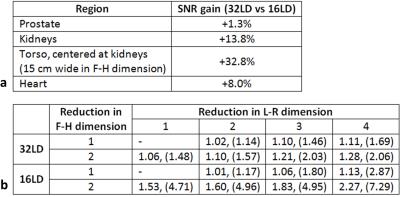
For a given excitation fidelity, the 32LD has 28% and 33% higher local SAR on average with single-spoke shimming inside kidneys and torso, respectively (Figure 2). SNR of both arrays inside various investigated organs are listed in Table 1.a, and SNR ratio of 32LD against 16LD are plotted along axial slices intersecting the prostate, kidneys and heart in Figure 3. In deeply situated regions (i.e. prostate) the 32LD did not have a significant SNR advantage, however in regions closer to surface 32LD outperformed the 16LD. The 32LD has 13.8% and 32.8% higher SNR inside the kidneys and torso, respectively.
Average and maximum values of g-factors for accelerations along L-R and F-H dimensions in a coronal slice intersecting the kidneys are listed in Table 1.b. Unlike 16LD, acceleration along the F-H dimension can be supported by the 32LD (i.e. g-factor≤1.2) owing to the placement of loop elements that direction. The 16LD has slightly better parallel imaging performance for accelerations ≤3 in L-R dimension.
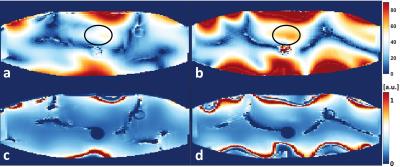
Experimentally acquired flip angle (top row) and SNR maps (bottom row) of 16LD (left column) and 32LD (right column) are shown in Figure 4.
Discussion/Conclusion
A 32-channel combined loop-dipole transceiver array was developed by combining 8 dipoles and 24 loops geometrically decoupled by careful element layout/placement. The 32LD has improved SNR near the surface of the body and parallel imaging performance along F-H dimension. The current 32-channel array design (32LD) did not improve parallel transmit performance compared to the 16LD within the investigated target anatomies, however an improved coil design with optimized loop dimensions and element placement is expected to provide additional benefits in transmit and SAR efficiency and is the topic of ongoing investigations.Acknowledgements
Supported by: NCI R01 CA155268, NIBIB P41 EB015894, S10 RR026783, WM Keck Foundation.References
1. Raaijmakers, A.J., et al., The fractionated dipole antenna: A new antenna for body imaging at 7 Tesla. Magn. Reson. Med., 2016, 75: 1366–1374. doi:10.1002/mrm.25596.
2. Erturk, M.A., et al., A 16-channel combined loop-dipole transceiver array for 7 Tesla body MRI. Magn Reson Med, 2016. doi:10.1002/mrm.26153.
3. Christ, A., et al., The Virtual Family--development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol, 2010. 55(2): p. N23-38.
4. Erturk, M.A., et al., Toward imaging the body at 10.5 tesla. Magn Reson Med, 2016. doi:10.1002/mrm.26487.
5. Erturk, M.A., et al., Development and evaluation of a multichannel endorectal RF coil for prostate MRI at 7T in combination with an external surface array. J Magn Reson Imaging, 2016. 43(6): p. 1279-87.
Figures