1199
Low-rank plus sparse tensor reconstruction for high-dimensional cardiac MRI1Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
The recently proposed general low rank tensor framework enabled a paradigm change, where data acquisition and image reconstruction are represented in a higher-dimensional space. The overall data space is sampled only as different states randomly coincide, which leads to data gaps. These gaps can introduce challenges in spatiotemporal fidelity for only low-rank- or only sparsity-based reconstructions. Here, a $$$\mathcal{L}+\mathcal{S}$$$ tensor decomposition is investigated, which offers a more robust solution as the sparse component captures updates on top of the overall dynamics represented in the low-rank component. A free-breathing, T1-sensitive cardiac MRI with real-time Cartesian data acquisition over multiple cardiac and inversion recovery phases is employed to investigate potentials for comprehensive cardiac MRI, including for instance late gadolinium scar cine imaging.
PURPOSE
Rapid continuous data acquisition and multidimensional image reconstruction has the potential to disentangle motion and contrast dynamics, which promises to reduce challenges in cardiac MRI and to use scan time more efficiently, e.g.$$$~$$$[1,2]. Whereas previous work employed sparsity constraints along each dimension separately$$$~$$$[2], tensors were more recently proposed to represent high-dimensional images in a unified way and assuming a low-rank tensor representation$$$~$$$[3,4,5]. However, low-rank tensor representation or sparsity constraints alone might be challenging, if rank-assumptions are violated or data gaps impair reconstruction fidelity. In this work, we investigate a low-rank plus sparse ($$$\mathcal{L}+\mathcal{S}$$$)$$$~$$$[6,7] tensor completion approach, which can represent multidimensional images more flexibly as the superposition of a common low-rank background tensor and dynamic innovations. This approach is applied to free-breathing, T1-sensitive cardiac MRI with real-time data acquisition over multiple cardiac and inversion recovery phases. This is motivated to enable late gadolinium enhancement (LGE) scar Cine imaging for efficient clinical practice.METHODS
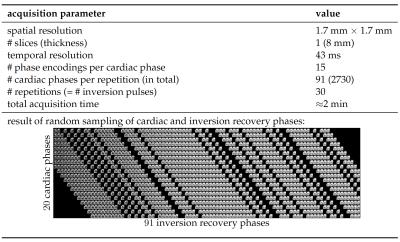
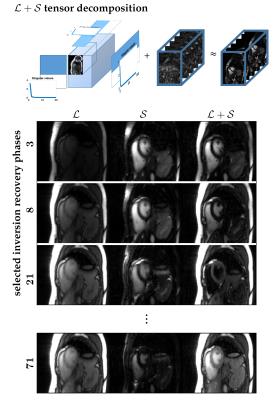
Repeated RF inversion was implemented in a 2D Cartesian real-time balanced steady state free precession prototype sequence$$$~$$$[8] which supports variable density incoherent sampling. 15$$$~$$$readout lines were acquired per time frame resulting in a frame rate of 43$$$~$$$ms and RF inversion was applied every 30$$$~$$$time frames (Fig.$$$~$$$1). Experiments were performed in two healthy volunteers on a clinical 3T MRI scanner (Magnetom Skyra, Siemens Healthcare GmbH, Erlangen, Germany) equipped with body and spine local RF receive arrays with up to 30$$$~$$$active elements. Continuously acquired 2D data were manually sorted into an undersampled 4D data tensor with 20$$$~$$$cardiac motion and 91$$$~$$$inversion recovery phases as dynamic dimensions. To this end, cardiac cycles were extracted from the real-time series and arranged as 'diagonals' in the space spanned by cardiac and inversion recovery phases (illustrated in Fig.$$$~$$$1). $$$\mathcal{L}+\mathcal{S}$$$ tensor reconstruction was performed by solving the following optimization problem:$$arg\!\min_{\mathcal{L},\mathcal{S}}{\left\|{\mathbf{E}\left(\mathcal{L}+\mathcal{S}\right)-\mathcal{Y}}\right\|}_2^2+\sum_{i=2}^{3}\lambda_i{\left\|{L_{(i)}}\right\|}_{\star}+\sum_{i=2}^{3}\mu_i{\left\|{\mathbf{T}S_{(i)}}\right\|}_{1},$$where $$$\mathcal{L},\mathcal{S}\in\mathbb C^{N_xN_y{\times}N_{ph}\times N_{TI}}$$$ represent the low-rank and sparse terms, respectively, of the image tensor $$$\mathcal{M}=\mathcal{L}+\mathcal{S}\in\mathbb{C}^{N_xN_y{\times}N_{ph}{\times}N_{TI}}$$$ (schematic in Fig.$$$~$$$2), $$$\mathcal{Y}\in \mathbb{C}^{N_x^{smp}N_y^{smp}{\times}N_{ph}^{smp}{\times}N_{coils}{\times}N_{TI}^{smp}}$$$ is the data tensor, $$$\mathbf{E}$$$ is the encoding operator, $$$\mathbf{T}$$$ is a sparsifying transform for the sparse component, and $$$\lambda_i,\mu_i$$$ are regularization parameters that weight the contributions of the nuclear-norm and $$$\ell$$$1-norm terms with respect to $$$\ell$$$2-norm data consistency. The subscript notation $$$L_{(i)}$$$ and $$$S_{(i)}$$$ refers to unfoldings along the $$$i$$$th dimension$$$~$$$[9]. For comparison, image reconstruction was also performed separately for only the low-rank (L-only) or only the sparsity (S-only) constraint, incorporating either the nuclear or $$$\ell$$$1-norm regularizer.RESULTS
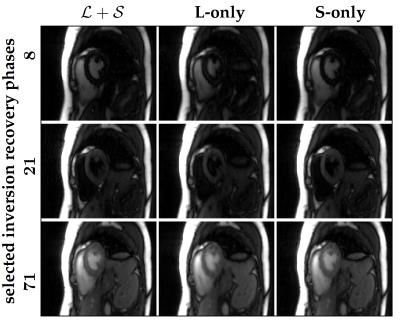
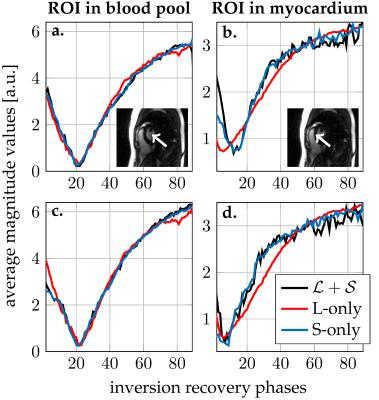
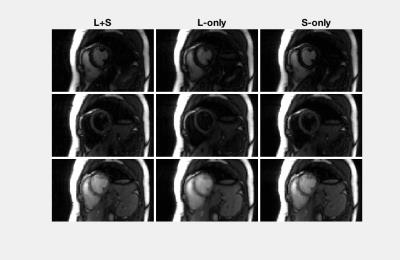
Figure$$$~$$$2 demonstrates the $$$\mathcal{L}+\mathcal{S}$$$ tensor decomposition for selected inversion recovery phases of the same cardiac phase. The low-rank background tensor $$$\mathcal{L} $$$ captures the main overall inversion recovery dynamics, whereas the sparse component $$$\mathcal{S}$$$ contains the innovations related to contrast changes on top of the main dynamics. This leads to an improved spatiotemporal resolution compared to the L-only or S-only reconstructions (Fig.$$$~$$$3). In particular, the L-only approach results in significant degradation, which becomes further apparent in the inversion recovery curve at the myocardium region (Fig.$$$~$$$4). Animation of one complete cardiac cycle at different contrasts (Fig.$$$~$$$5) shows slight residual aliasing also for $$$\mathcal{L}+\mathcal{S}$$$.DISCUSSION
The general tensor framework encompasses a paradigm change, where data acquisition and image reconstruction are represented in a higher-dimensional space. However, sampling in the higher-dimensional space usually results in data gaps (Fig.$$$~$$$1) that introduce challenges for low-rank-based or sparsity-based reconstructions. A typical L-only or S-only approach will present a solution that implicitly interpolates the data gaps at the expense of spatiotemporal resolution$$$~$$$(Figs.$$$~$$$3-5). The proposed $$$\mathcal{L}+\mathcal{S}$$$ tensor completion approach offers a more robust solution as data gaps can be represented as outliers in the model, which supports higher spatiotemporal resolutions (Figs.$$$~$$$3-5). One limitation in the current approach is that organ motion is not managed appropriately by $$$\mathcal{L}+\mathcal{S}$$$, which is causing residual aliasing artifacts (Fig.$$$~$$$5). Next steps will include employing a motion model in
the $$$\mathcal{L}+\mathcal{S}$$$ reconstruction, as demonstrated
in$$$~$$$[10]. Likewise, different sampling patterns and timing
parameters, so as to best populate the higher-dimensional space is of
further interest. Many clinical protocols comprise inversion recovery for LGE imaging, which requires a pre-scan to determine the best inversion time for targeted contrast behavior. The presented Cartesian acquisition has the potential for establishing efficient comprehensive cardiac exams, including LGE scar Cine imaging or the recently presented high quality T1 assessment [5].
CONCLUSION
$$$\mathcal{L}+\mathcal{S}$$$ tensor completion can provide a robust, unbiased and flexible approach to multidimensional reconstruction of cardiac phases at different inversion recovery states with high temporal resolution of cardiac and contrast dynamics. The methodology presented here can also be readily applied to multidimensional imaging of different organs such as the liver or kidneys.
Acknowledgements
We thank the NIH for supporting our work, NIH grant P41EB017183.References
1. J. Pang, B. Sharif, Z. Fan, X. Bi, R. Arsanjani, D. S. Berman, and D. Li, "ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function", Magn. Reson. Med., 2014, pp. 1208 -1217.
2. L. Feng, L. Axel, H. Chandarana, K. T. Block, D. K. Sodickson, and R. Otazo. "XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing," Magn. Reson. Med., 2016, pp. 775-788.
3. J. D. Trzasko and A. Manduca, "A unified tensor regression framework for calibrationless dynamic, multi-channel MRI reconstruction", in Proc. Int. Soc. Magn. Reson. Med., 2013, p. 603.
4. A. G. Christodoulou and Z.-P. Liang, "3D dynamic T1 mapping of the myocardium using a time-varying subspace", in Proc. Int. Soc. Magn. Reson. Med., 2015, p. 2614.
5. A. G. Christodoulou, J. L. Shaw, B. Sharif, and D. Li "A general low-rank tensor framework for high-dimensional cardiac imaging: Application to time-resolved T1 mapping", in Proc. Int. Soc. Magn. Reson. Med., 2016, p. 867
6. E. J. Candès, X. Li, Y. Ma, and J. Wright. “Robust Principal Component Analysis?”, Journal of ACM, 2009, pp 1-37.
7. R. Otazo, E. Candes, and D. K. Sodickson, “Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components”, in Magn Reson Med, 2015, pp.1125-1136.
8. G. Vincenti, et al. "Compressed sensing single-breath-hold CMR for fast quantification of LV function, volumes, and mass", JACC: Cardiovascular Imaging 7.9, 2014, pp. 882-892.
9. T. G. Kolda and B. W. Bader, "Tensor decompositions and applications", SIAM Review, 2009, pp. 455-500.
10. R. Otazo, et al. "Motion-guided low-rank plus sparse (L+ S) reconstruction for free-breathing dynamic MRI", in Proc. Int. Soc. Magn. Reson. Med., 2014, p. 742.Figures