1191
Proton Density and R2* Estimation of Neonatal Lung Parenchyma During Free Breathing with UTE MRI1Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Center for Pulmonary Imaging Research, Division of Pulmonary Medicine and Department of Radiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 3Department of Physics, Washington University in St. Louis, St. Louis, MO, United States, 4Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, 5Department of Physics, University of Cincinnati, Cincinnati, OH, United States, 6Perinatal Institute, Division of Neonatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
Synopsis
The majority of patients in the neonatal intensive care unit (NICU) have pulmonary morbidities, yet little is known about the underlying parenchymal structure. We quantify parenchymal proton density and R2* in the lungs of quiet breathing, non-sedated neonates in the NICU using a multi-echo 3D radial UTE MRI. Results indicate that lung proton density decreases as expected with lung inflation, while R2* increases. A positive relationship between gravitational dependence and tissue density is also apparent, while R2* decreases in more gravitationally dependent regions. Overall, our findings support a negative relationship between tissue density and R2* in the neonatal lung.
Purpose
Recent work has demonstrated the feasibility for ultrashort echo time (UTE) MRI of the lung in quiet breathing, non-sedated, neonatal intensive care unit (NICU) patients1, which is of particular interest due to the elevated risk in this population of exposure to ionizing radiation from x-ray computed tomography (CT). Parenchymal density and R2* are biophysical parameters which are important for designing optimal neonatal lung MRI techniques, and may potentially contain clinically useful information for diagnosing and monitoring pathology such as microstructural abnormalities including alveolar simplification due to bronchopulmonary dysplasia (BPD). Here, multi-echo 3D UTE MRI is used to quantify parenchymal tissue density and R2* in the lungs of NICU patients during quiet tidal breathing, utilizing the self-navigation properties of the acquisition sequence to retrospectively reconstruct separate images at end-tidal inspiration and expiration2.Methods
All studies were approved by our institutional review board, were HIPAA compliant, and were conducted with parental consent. 11 neonatal patients underwent MRI in the NICU (post-menstrual ages 36-43 weeks at date of imaging) on a 1.5T MRI scanner3 designed specifically for neonatal imaging, using a body coil. A 3-D radial UTE MRI sequence1, was modified to acquire each radial view at 4 different echo times (TE = 0.20ms, 0.95ms, 1.70ms, 2.45ms, 50,000-75,000 projections per TE, 1.4mm isotropic image resolution). 2 adults also underwent MRI on a conventional 1.5T scanner using the same imaging sequence with an 8-channel coil and slightly different parameters (TE = 0.09ms, 0.59ms, 1.09ms, 1.59ms, 25,000 projections per TE, 2.0mm isotropic image resolution). The center of k-space acquired with each view was used for self-navigated retrospective respiratory gating2, and end-tidal inspiration and expiration images (50% acceptance windows) were reconstructed at each TE. Least squares estimates of R2* and spin density (M0) were estimated at both respiratory phases from
$$S_k(r)=M_0e^{TE_k(2\pi i\Delta B_0(r)-R_2^*(r))}, \space\space\space k=1,2,3,4$$
, where Sk and TEk are the measured signal and echo times at the kth echo, ΔB0 is the field off-resonance and r varies over all voxels. Parenchymal spin density was estimated by normalizing M0 to adjacent muscle tissue signal (expressed as % of muscle), motivated by the assumption that lung and muscle tissues are expected to be similarly T1-weighted1,4. Average whole lung estimates of tissue density and R2* are compared between inspiration and expiration, using a paired t-test to evaluate statistical significance. Additionally, anterior-posterior (AP) relationships in these parameters are explored by dividing the segmented lung volumes into thirds along the AP axis (anterior/middle/posterior regions). Due to the coil sensitivity variations, proton density was not analyzed in adult data.
Results
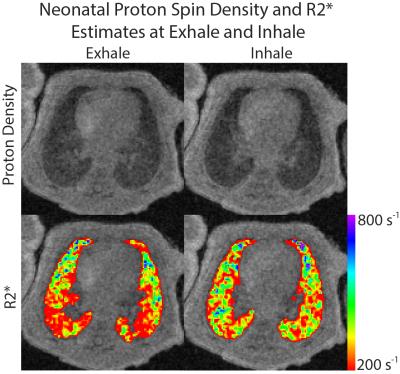
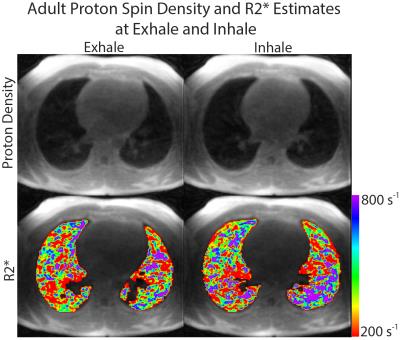
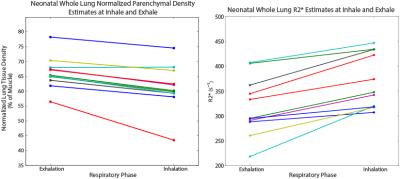
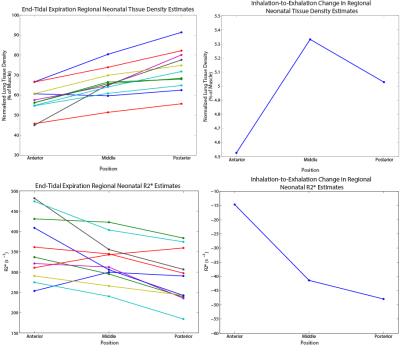
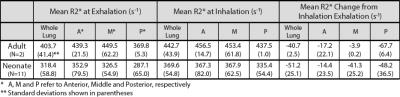
Representative images of lung tissue density and parametric maps of R2* are shown at both inspiration and expiration in Figure 1 and Figure 2 for a neonatal and adult subject, respectively. The larger R2* values at inspiration relative to expiration are generally apparent from these images in both subjects, along with a negative AP gradient in R2* in the neonate, particularly at end-expiration. This latter relationship is not as clear in the adult images. The plots in Figure 3 demonstrate whole lung tissue density and R2* at expiration and inspiration in the neonatal cohort. As expected, tissue density decreases as the lung inflates (p = 1.8x10-4). However, R2* shows the opposite trend, increasing with lung inflation (p = 2.5x10-5). Regional AP trends in the estimated parameters at end-expiration, along with the differences between end-tidal inspiration and expiration, are shown in Figure 4 for neonates. Figure 4 demonstrates that proton density tends to increase in posterior lung regions at end-expiration, and decrease at end-inspiration as expected due to gravity dependence. Figure 4 also supports the finding that R2* increases as proton density decreases especially in the posterior, gravity-dependent region. Mean regional R2* estimates are presented in Table 1 for both adults and neonates. These neonatal trends are also seen in adults; however, R2* in adults is consistently larger.Discussion and Conclusion
We present initial results of proton density and R2* measures in the lungs of free breathing neonates, and compare these data to similar estimates in adults. These results support a negative relationship between proton density and R2* that is maintained for both end-tidal and end inspiratory lung inflation. R2* also appears to be smaller in the lungs of neonates relative to adults, although more adult data is required to verify this finding. There is a wide range of measured baseline tissue densities and R2* values in this neonatal cohort. Determination of the source of these differences may have a clinically useful physiologic basis and is an important topic for further research.Acknowledgements
The authors would like to acknowledge financial support from The Hartwell Foundation, GE Healthcare, The Perinatal Institute at Cincinnati Children’s Hospital Medical Center, and NIH P01 HL070831.References
1. Hahn AD, Higano NS, Walkup LL, Thomen RP, Cao X, Merhar SL, Tkach JA, Woods JC, Fain SB. (2016) Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging. doi: 10.1002/jmri.25394
2. Higano NS, Hahn AD, Tkach JA, Cao X, Walkup LL, Thomen RP, Merhar SL, Fain SB, Woods JC (2016) Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med. doi: 10.1002/mrm.26212
3. Tkach JA, Merhar SL, Kline-Fath BM, Pratt RG, Loew WM, Daniels BR, Giaquinto RO, Rattan MS, Jones BV, Taylor MD, Tiefermann JM, Tully LM, Murphy EC, Wolf-Severs RN, LaRuffa AA, Dumoulin CL (2014) AJR Am J Roentgenol. 202(1):W95-W105. doi: 10.2214/AJR.13.10613.
4. Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C (2004) Musculoskeletal MRI at 3.0T: relaxation times and image contrast. Am J Roentgenol 183:343–351. doi: 10.2214/ajr.183.2.1830343
Figures