1188
Investigating regional pulmonary structure-function relationships using hyperpolarized 129Xe MRI and ultra-short echo-time MRI1Center for Pulmonary Imaging Research, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Pulmonology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
Synopsis
We have quantified the extent of ventilation impairment in lungs due to specific pathologies associated with cystic fibrosis (CF) lung disease using ultrashort echo-time (UTE) MRI to identify structural abnormalities and hyperpolarized (HP) 129Xe MRI to identify ventilation deficits. We found that bronchiectasis demonstrates the best correlation with lung function decline, as measured by the percent predicted forced expiratory volume in 1 second (FEV1% predicted) and demonstrated the greatest deficit in HP 129Xe signal within corresponding defective regions. However, the greatest volume-percentage of defects identified were due to mucus plugging.
Introduction
Recent advances in ultra-short echo (UTE) techniques have made MRI rival the resolution and diagnostic quality of x-ray CT1, with regional identification of specific cystic fibrosis (CF) pathologies, like mucus plugging and bronchiectasis2. Likewise, hyperpolarized (HP) gas MRI of lung has demonstrated the ability to quantify regional ventilation in lungs with sensitivity higher than clinically-accepted spirometric techniques3,4. However, a gap exists in relating regional structural abnormalities to functional deficits. The purpose of our study was to bridge this gap by identifying specific CF pathologies via UTE MRI and to relate those structural abnormalities to regional function, via HP 129Xe MRI. These pulmonary structure-function relationships may aid in development of regionally-targeted and/or pathology-specific treatments of CF lung disease, and would be useful in longitudinal studies to understand disease progression.Methods
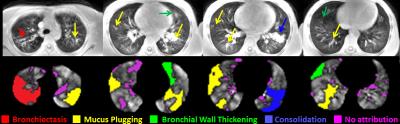
Eleven pediatric CF patients (ages 11.5 ± 2.5, range 7-16) were recruited for this study. Each subject underwent a free-breathing UTE MRI scan (gated acquisition at end expiration using an image navigator; FA=5°, TR/TE=5.8ms/0.2ms, voxel size=1.39×1.39×4mm3) and a single-breath HP 129Xe MRI scan within the same imaging session (inspiration=1/6 total lung capacity, from functional residual capacity; FA=9-14°; TR/TE=8ms/4ms; matrix=52-96×96-144; voxel=3×3×15 mm3; duration <16s). The HP 129Xe images were analyzed for regions of defective ventilation (defined as voxel signal <60% of the whole-lung signal mean) and quantified as a ventilation defect percentage (VDP). Using this threshold to define defects, the mean HP 129Xe signal contained within each defect was also quantified. HP 129Xe images were then regionally matched to UTE images in order to identify the following structural pathologies potentially responsible for obstructed ventilation: bronchiectasis, mucus plugging, bronchial wall thickening, ground glass opacities, air trapping, and/or consolidation (Figure 1). Defects with no apparent corresponding pathology were also quantified. The number and volume percentage of specific defects attributable to each pathology is reported as well as the average HP 129Xe signal contained within pathology-specific regions. Whole-lung VDP and patient FEV1% predicted (forced expiratory volume in 1 second) were also compared.Results
The mean VDP for all subjects was 19.0%±7.3% and mean FEV1% predicted was 88.0%±15.8% (Pearson correlation r=-0.32, p=0.33). Of all identified defect regions, 49.7% were attributable to pathology seen in UTE MR images. Of all attributed pathologies, bronchiectasis was found to correspond to the lowest average HP 129Xe signal (26.2%±7.8%, expressed as percentage of lung signal mean), and mucus plugging was responsible for the greatest number of identified defects (patient mean 5.3±3.8, out of 7.9±5.0 total defects). Individual statistics for each pathology are given in Figure 2. Around half of the defects identified were not associated with a specific pathology. Defect percentage due to bronchiectasis was found to correlate best with patient FEV1% predicted decline (r=-0.34, p=0.31) and was comparable to the correlation between FEV1% predicted and whole-lung VDP (r=-0.32, p=0.33). Regions of the lung which did not exhibit ventilation defects averaged 118%±9% of the whole-lung mean HP 129Xe signal.Discussion
This method provides an avenue for identification of individual structural abnormalities that are responsible for regional ventilation deficits. UTE MRI was able to visualize all of the typical CF-specific structural abnormalities, yet only half of the identified defects were attributable to identified pathology, potentially due to finite image resolution. In early CF lung disease, bronchiectasis correlated best with clinical lung function decline as indicated by VPD and FEV1% predicted, and defects associated with bronchiectasis demonstrated the lowest 129Xe signal. While mucus plugging was responsible for the greatest number and volume of defects, individual defects caused by bronchiectasis were much larger in size and correlated better with lung function decline. Notably, bronchiectasis is generally considered permanent lung remodeling, while mucus plugging is potentially reversible through treatment.Conclusions
Quantifying the extent of lung function decline due to specific structural pathologies in CF lung disease is feasible using UTE MRI and HP 129Xe MRI. In these 11 subjects, bronchiectasis correlated best with spirometrically measured obstruction, and mucus plugging was responsible for the largest number and volume of attributed defects.Acknowledgements
No acknowledgement found.References
1. Roach DJ, Crémillieux Y, Serai SD, Thomen RP, Wang H, Zou Y, Szczesniak RD, Benzaquen S, Woods JC. Morphological and quantitative evaluation of emphysema in chronic obstructive pulmonary disease patients: A comparative study of MRI with CT. Journal of Magnetic Resonance Imaging 2016:n/a-n/a.
2. Roach DJ, Crémillieux DY, Fleck DRJ, Brody DAS, Serai DSD, Szczesniak PRD, Kerlakian MS, Clancy DJP, Woods DJC. Ultrashort Echo-Time Magnetic Resonance Imaging Is a Sensitive Method for the Evaluation of Early Cystic Fibrosis Lung Disease. Annals of the American Thoracic Society;0(ja):null.
3. Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. Journal of Cystic Fibrosis.
4. Walkup LL, Thomen RP, Akinyi TG, Watters E, Ruppert K, Clancy JP, Woods JC, Cleveland ZI. Feasibility, tolerability and safety of pediatric hyperpolarized 129Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol 2016;46(12):1651-1662.