1181
Toward hybrid MR thermometry in aqueous and adipose tissue using simultaneous dual contrast weighting with double echo RARE imaging1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany, 3MRI.TOOLS GmbH, Berlin, Germany
Synopsis
Proton resonance frequency (PRF) shift is the most common MR thermometry (MRTh) technique for water based tissue. For adipose tissue MRTh exploits the temperature dependent relaxation times T1 and T2. Hybrid methods that allow simultaneous acquisition of water and fat based thermometry are of great clinical need. Recognizing this need this work investigates a hybrid temperature mapping technique, designated as 2in1-Thermometry, which combines simultaneous PRF and T2-based temperature mapping using simultaneous dual contrast weighting with double echo RARE imaging.
Purpose
MR-Thermometry (MRTh) is key for guidance and supervision of thermal therapies1-3 and beneficial for monitoring and improving MR safety4-5. Proton resonance frequency (PRF) shift is commonly used for MRTh of aqueous tissue. For MRTh of adipose tissue temperature dependence of T1 and T26-7 relaxation times is exploited. Hybrid methods that allow simultaneous acquisition of water and fat based thermometry are of great clinical need8. Recognizing this need this work proposes a hybrid temperature mapping technique, designated as 2in1-Thermometry, which combines simultaneous PRF and T2-based temperature mapping using simultaneous dual contrast weighting with double echo RARE imaging. 2in1-Thermometry is carefully validated in phantom heating experiments and benchmarked against conventional gradient echo (GRE) based PRF thermometry. Preliminary results of RARE based ΔT2 thermometry in bovine suet are validated against fiber optic temperature sensor readings.Methods
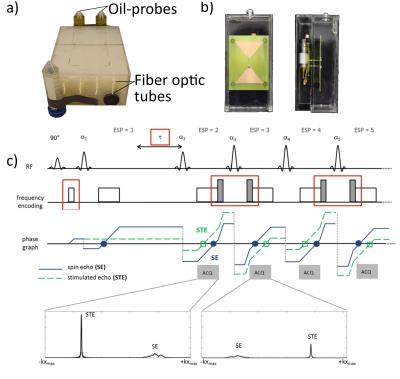
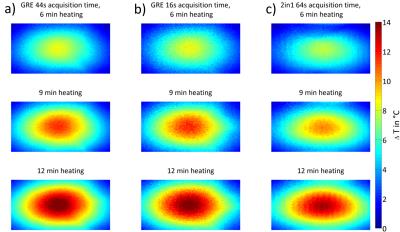
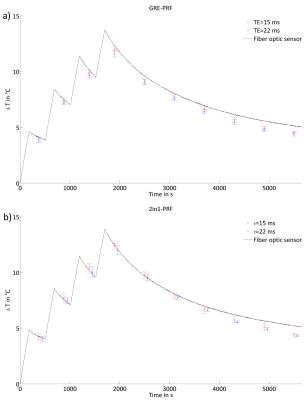
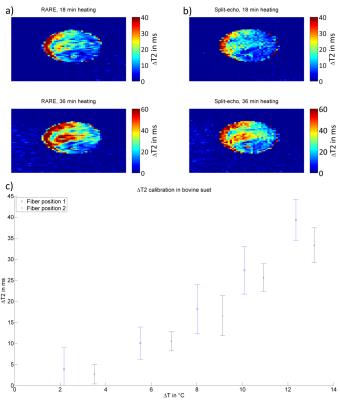
2in1-RARE imaging supports two contrasts in one acquisition by independent sensitization of stimulated echoes (STE) and spin echoes (SE)9. For PRF-MRTh an evolution time is incorporated between the initial excitation and the first refocusing pulse to generate T2*-weighting for the SE magnetization10. The STE-image is not affected by this evolution time and is acquired for two TEs permitting simultaneous T2-based MRTh assuming a mono-exponential T2-decay for fatty tissue11 (Fig.1c). Radiofrequency (RF) heating was performed at f=300MHz using a bow tie dipole-antenna fed with an external RF-power amplifier12 (Fig.1b). MR images were acquired at 3.0T13. To examine the fidelity of PRF-MRTh of 2in1-RARE, heating experiments were performed in phantoms and benchmarked against GRE based PRF. For this purpose a uniform agarose phantom was heated four times for three minutes (Pavg=70W at the antenna). B0-drift correction was performed using oil samples placed around the phantom. To study the feasibility of T2 based MRTh, heating experiments of fatty tissue were performed using conventional RARE and split-echo RARE11,14 as a precursor of 2in1-RARE. For this purpose an agarose phantom including an insert containing bovine suet was heated for 6x6min (Pavg=70W at the antenna). Before heating and directly after each heating period RARE and split-echo RARE were performed to calculate a ΔT2-map based on a mono-exponential decay (TE1=17ms, TE2=52ms). Temperature maps were calculated based on T2 changes versus the reference image. MR-temperature maps were compared to measurements of fiber optic sensors15 placed inside the phantom.Results
PRF temperature maps calculated from 2in1-RARE and GRE are shown in Fig.2. The match between the maps demonstrates the feasibility of PRF based thermometry of 2in1-RARE using a slightly longer acquisition time to ensure a similar SNR. The temperature rise over time is displayed in Fig.3 for one fiber optic sensor position. The difference between the fiber optics data and the MR based temperature averaged in a region of interest (ROI) around the sensor position was defined as the accuracy (dT) of the temperature mapping technique. These accuracies were averaged for all four sensor positions and all MR measurements for a final accuracy of dT=(0.5±0.2)°C for GRE based PRF-MRTh and dT=(0.6±0.2)°C for 2in1-RARE based PRF-MRTh. The temperature dependent ΔT2 measurements of split-echo RARE in bovine suet tissue were comparable to ΔT2 values acquired using RARE (Fig.4). In comparison to fiber optic sensor readings in the bovine suet sample, RARE based temperature dependent ΔT2 changes showed a linear dependency with ΔT changes11 (Fig.4d).Discussion
The presented data demonstrate that 2in1-RARE based PRF thermometry is feasible and supports a temperature mapping fidelity competitive with GRE based PRF-MRTh. To take our developments to the next level the presented preliminary T2 based thermometry for RARE and spilt-echo RARE will be incorporated into 2in1-RARE. However the echo separation in 2in1-RARE leads to signal dephasing affected not only by T2 dephasing and consequently smaller T2 changes compared to RARE are presumed. A T2 preparation module16 will be added to the 2in1-RARE sequence and is expected to produce similar results to RARE and split-echo RARE, which showed good linearity (ΔT2/ΔT) as compared to fiber optic readings. The proposed 2in1-RARE-MRTh is especially beneficial for temperature monitoring applications where the traditional gradient echo and echo planar imaging based approaches are compromised bysusceptibility artifacts and image distortion. For the proposed 2in1-RARE-MRTh approach temperature sensitization can be varied from zero, which is not feasible with conventional gradient echo based PRF-MRTh.Conclusion
A spin echo based pulse sequence - 2in1-RARE thermometry – was presented that offers the potential to simultaneously acquire two images with different contrasts (T2* and T2). This enables the simultaneous calculation of temperature maps in adipose and water-based tissue which is a remaining challenge for GRE based thermometry.Acknowledgements
This work was supported in part (L.W., T.N.) by the German Federal Ministry of Education and Research, “KMU-innovativ”: Medizintechnik 13GW0102.References
1 Quesson B, De Zwart J and Moonen CTW, JMRI, 2000. 12(4):525-533
2 Moonen CTW, Medica Mundi, 2000. 44:34-42
3 Wust P, et al., Lancet Oncol, 2002. 3(8):487-497
4 Murbach M, et al., MRM, 2014. 71(1):421-31
5 Van Rhoon GC, et al., Eur Radiol, 2013. 23(8):2215-27
6 Rieke V and Pauly KB, JMRI, 2008. 27:376-390
7 Winter L, et al., Int J Hyperthermia, 2015. 32(1):63-75
8 Todd N, et al., MRM, 2014. 72(3):793-9
9 Fuchs K, et al., MRM, 2014. 72(6):1590-8
10 Streicher MN, et al., MRM, 2014. 71(2):524-533
11 Baron P, et al., MRM, 2014. 72(4):1057-1064
12 Winter L, et al., Radiat Oncol, 2015. 10(1):201
13 Verio, Siemens, Erlangen, Germany
14 Schick F, MRM, 1997. 38(4):638-644
15 Omniflex, Neoptix, Québec, Canada
16 Brittain JH, MRM, 1995. 33(5):689-696
Figures