1178
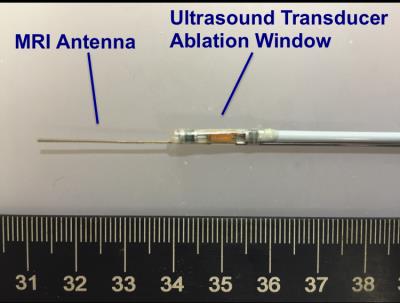
A Combined Intravascular MRI Endoscope and Intravascular Ultrasound (IVUS) Transducer for High-Resolution Image-Guided Ablation1Russell H. Morgan Department of Radiology, Johns Hopkins University, Baltimore, MD, United States, 2Electrical and Computer Engineering, Johns Hopkins University, Baltimore, MD, United States, 3Acoustic MedSystems, Inc, Champaign, IL, United States
Synopsis
An intravascular MRI (IMRI) loopless antenna is combined for the first time with an intravascular water-cooled ultrasound ablation transducer as a possible tool for providing high-resolution MRI-guided ablations of pathological tissue via intravascular access. High resolution anatomical MRI, and real-time MRI thermometry were used to monitor ablation delivery in phantoms and tissue specimens. Results show that IMRI can guide IVUS-mediated directional ablation with minimal image artifacts. This permits the monitoring of thermal dose and therapy titration while minimizing potential thermal damage to the vessel wall.
Purpose
Vascular invasion of critical vessels in some cancers (such as the superior mesenteric artery in locally advanced pancreatic adenocarcinoma) often renders tumors inoperable, even in the absence of metastases.1 Many common palliative and/or curative treatments in clinical trials employ percutaneous thermal ablation (radiofrequency, RF; microwave; cryoablation; etc.) or extracorporeal high-intensity focused ultrasound ablation (HIFU).2-4 These are prone to complications arising from imprecise ablation delivery, vascular injury, lack of access to deeply-seated organs and/or problems associated with blood flow or physiological motion5. Because intravascular MRI (IMRI) could provide high-resolution therapy guidance,6-9 and because, unlike RF-, cryo- and microwave-ablation systems, intravascular ultrasound can heat distant tissue directly without relying on conduction, we combined an IMRI antenna with an ultrasound ablation probe to provide directional ablation of soft tissue from a nearby blood vessel. The goal is to achieve targeted perivascular ablation with minimal injury to the intervening vessel wall. We demonstrate high-resolution MRI-guided directional targeting of perivascular ultrasound ablation with the device, as monitored in real-time by MRI thermometry in phantoms and organ specimens.Methods
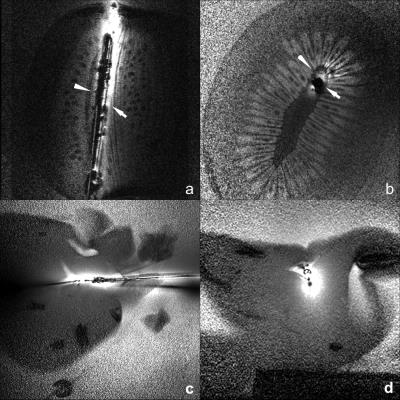
A home-built 0.7mm-diameter, 42 mm-whip, IMRI loopless antenna6,7 was combined with an ultrasound ablation catheter (Acoustic MedSystems, Inc; Fig.1). The ultrasound catheter comprised a flat 6.26 MHz transducer (60° energy-deposition arc) in a 2.3mm water-cooled jacket. The antenna whip-cable junction was aligned with the intravascular ultrasound ablation window. MRI was performed on a Philips Medical Systems, 3T Achieva system coupled with a modified Philips Sonalleve MR thermometry graphic user interface (GUI)10. Images were processed with a radial-dependent spatial filter to correct for the antenna’s ~1/r SNR profile. To test whether the presence of the IVUS transducer and leads compromised antenna performance, MRI was done in a kiwi fruit using a turbo spin-echo (TSE) sequence (echo-time, TE =30ms; repetition time, TR =3s; resolution =200μm).
In vitro studies included muscle and freshly harvested pig liver accessed percutaneously and via a portal vein, respectively. Specimens were immersed in 35°C saline (0.35%) for ablation. MRI was performed (3D balanced fast-field echo, bFFE; TR/TE=6.1/2.3ms; resolution =300μm; FOV=150x150x12 mm3; flip-angle=20°), followed by ultrasound ablation (6 min @7 Wacoustic) during which the catheter was water-cooled (22°C). During ablation, MR thermometry (echo-planar proton resonant frequency shift11 imaging; TR/TE =28/16ms, 0.9x0.9x6mm3, 1.2 s/frame) was performed in real-time to monitor temperature change and thermal dose. The ablation was terminated upon achieving a ~1cm ablation lesion. Lesions were identified by gross anatomy post-MRI, and correlated with thermal dose as identified by MR thermometry.
Results
IMRI of a kiwi fruit and pig liver (Fig. 2) show negligible artifact from the ultrasound transducer other than a signal void due to the absence of protons.
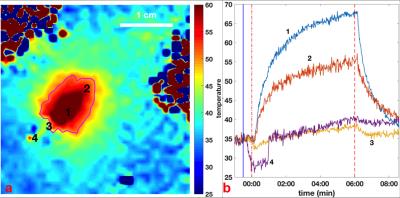
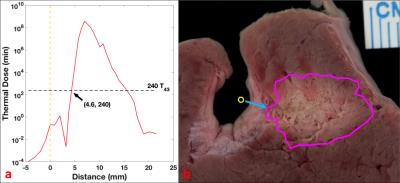
Fig. 3a shows an MRI thermal map acquired at the end of a 6-min ablation with four regions of interest (ROIs) annotated, and a photograph of the corresponding anatomical section shows the lesion (Fig. 4b; magenta circle). Temperature changes during the whole process of treatment are shown in Fig. 3b. Temperatures in the target ROIs away from the vessel and probe, increase monotonically during ablation up to 67°C. Meanwhile, temperatures closest to the vessel are relatively constant or initially decrease upon commencement of circulation of the cooling water, and remain <40°C for the entire procedure. Contours for a thermal dose delivered equivalent to 240min at 43°C (which demarcates coagulation),12 as calculated from the thermal maps, are also plotted in Fig.(3a,4b; magenta circle). The thermal dose vs. distance along the axis of peak energy delivery (perpendicular to the transducer’s long axis), is plotted in Fig.4a. The 240-min-43°C threshold exhibits a 4.6-mm-wide non-coagulation area outside of the transducer, wherein vessel wall is expected to be spared during ablation.
Discussion
We presented a new thermal ablation system comprised of a high-resolution IMRI probe and an ultrasound ablation catheter. The combination provided artifact-free MRI for anatomical targeting,13 along with thermometry for documenting thermal dose. Importantly, unlike other ablation methods that typically generate burns at the points of contact, the use of intravascular ultrasound avoided thermal injury to vessel wall adjacent to the probe, suggesting potential applications for ablating perivascular tumors. Water-cooling of the transducer is likely an important factor limiting temperature rise adjacent to the probe. Although the present study was limited to tissue specimens, the presence of blood flow and perfusion in vivo should enhance this protective effect.Acknowledgements
Grant support: NIH R01 EB007829.References
1. Buchs NC, Chilcott M, Poletti PA, Buhler LH, and Morel P. Vascular invasion in pancreatic cancer: Imaging modalities, preoperative diagnosis and surgical management. WJG. 2010;16(7):818-831.
2. Petrou A, Moris D, Tabet PP, Richards BDW, and Kourounis G. Ablation of the locally advanced pancreatic cancer: An introduction and brief summary of techniques. JBUON. 2016;21(3):650-658.
3. Linecker M, Pfammatter T, Kambakamba P, and DeOliveira ML. Ablation Strategies for Locally Advanced Pancreatic Cancer. Dig. Surg. 2016;33:351–359.
4. Ertürk MA, Hegde SS, and Bottomley PA. Radiofrequency Ablation, MR Thermometry, and High-Spatial-Resolution MR Parametric Imaging with a Single, Minimally Invasive Device. Radiology. 2016;doi: http://dx.doi.org/10.1148/radiol.2016151447.
5. Adams MS, Scott SJ, Salgaonkar VA, Sommer G and Diederich CJ. Thermal therapy of pancreatic tumours using endoluminal ultrasound: Parametric and patient specific modeling. International Journal of Hyperthermia. 2016;32(2):97-111.
6. El-Sharkawy AMM, Qian D, and Bottomley PA. The performance of interventional loopless MRI antennae at higher magnetic field strengths. Med. Phys. 2008;35(5):1995-2006.
7. Sathyanarayana S and Bottomley PA. MRI endoscopy using intrinsically localized probes. Med. Phys. 2009;36(3):908-919.
8. Sathyanarayana S, Schär M, Kraitchman DL, and Bottomley PA. Towards Real-Time Intravascular Endoscopic Magnetic Resonance Imaging. JACC: Cardiovascular Imaging. 2010;3(11):1158-1165.
9. Hegde SS, Zhang Y, and Bottomley PA. Acceleration and Motion-Correction Techniques for High-Resolution Intravascular MRI. MRM. 2015;74:452–461.
10. Ellens N, Partanen A, Ghoshal G, Burdette E and Farahani K. Integration of Interstitial High Intensity Therapeutic Ultrasound Applicators On a Clinical MRI-Guided High Intensity Focused Ultrasound Treatment Planning Software Platform. Med. Phys. 2015;42:3264.
11. Rieke V, and Pauly KB. MR Thermometry. J Magn Reson Imaging. 2008;27(2):376–390.
12. McDannold NJ, King RL, Jolesz FA, and Hynynen KH. Usefulness of MR Imaging–Derived Thermometry and Dosimetry in Determining the Threshold for Tissue Damage Induced by Thermal Surgery in Rabbits. Radiology. 2000;216:517-523.
13. Wang G, Erturk MA, Hegde SS, and Bottomley PA. Automated classification of vessel disease based on high-resolution intravascular multi-parametric mapping MRI. Proceedings of the International Society for Magnetic Resonance in Medicine . Scientific Meeting and Exhibition International Society for Magnetic Resonance in Medicine Scientific Meeting and Exhibition 2015. 2015:1659-.
Figures