1172
Efficiency improvement in multi-point MR acoustic radiation force impulse imaging1Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 2Department of Radiology, Stanford University, 3Department of Radiology and Imaging Sciences, University of Utah
Synopsis
Focused ultrasound (FUS) treatment endpoint can potentially be evaluated using MR acoustic radiation force impulse (MR-ARFI) imaging, which can non-invasively and with very low energies interrogate tissue mechanical properties. In this work we investigate two ways of improving the efficiency of a previously published multi-point (MP)-MR-ARFI pulse sequence which simultaneously measure ARFI displacement and PRFS temperature maps. We investigate view-sharing of higher k-space frequencies from FUS-OFF images to FUS-ON images, and focusing in multiple positions during a single motion encoding gradient lobe as ways to speed up acquisition. Combining these methods full 3D MP-displacement maps could be achieved in a clinically acceptable time of 30-60s.
Purpose
MR-guided focused ultrasound (FUS) treatment endpoint can be evaluated by accumulated thermal dose (TD) or contrast enhanced imaging. While all tissues can be considered ablated once a TD of 240 CEM43 has been accumulated, it has been shown that certain tissues such as the brain can lose viability at substantially lower TD(1,2). Targeting 240 CEM43 for these tissues therefor poses unnecessary risk for off-focal tissue damage. MRI contrast agent administration can currently only be performed post-treatment, and is therefore not an option for dynamic endpoint evaluation. A possible alternative is to evaluate treatment endpoint using MR acoustic radiation force impulse (MR-ARFI) imaging, which can interrogate tissue mechanical properties such as stiffness using very low energy. In this work we improve the efficiency of a novel volumetric multi-point (MP)-MR-ARFI method, which simultaneously measures tissue displacement and PRFS MR thermometry(3,4).Methods
Bi-polar motion encoding
gradients (MEG) were implemented in a 3D GRE segmented EPI pulse sequence. Optical
triggers are emitted at the start of the second MEG lobe to synchronize the FUS
pulses with the MEG. A single image acquisition with FUS-OFF (i.e. power=0W)
was interleaved at the TR level with multiple image acquisitions with FUS-ON (power>0W),
with different focal spot location for each FUS-ON image. Since any phase
change in the FUS-OFF image will be due solely to heating during the multiple FUS-ON
images, it can be used to calculate the PRFS temperature maps. The phase change
in the FUS-ON images will be due to both PRFS and tissue displacement during
the MEG. Complex phase subtraction of the FUS-OFF image from the FUS-ON images
will result in a phase-change map solely due to tissue displacement. The TR-level
interleaving of the FUS-OFF and FUS-ON images will give a more accurate PRFS estimate
than if the full FUS-OFF and FUS-ON images were acquired sequentially.
In this work we present initial
results of changes in tissue stiffness after FUS ablation, as well as investigate
two approaches to improve the efficiency of the MP-MR-ARFI acquisition. 1) The acquisition
time can be reduced by utilizing a view sharing-type (a.k.a Keyhole) reconstruction
where a portion of the higher frequencies in the ky-phase encoding and/or kz-slice
encoding directions are only acquired once for the FUS-OFF image and is then “shared”
with the FUS-ON images. 2) Multiple focal spot locations can be acquired during
a single image by emitting multiple optical triggers during a single MEG lobe.
All MR imaging was performed on a 3T scanner
(Siemens PrismaFit, Germany) with a single-channel RF loop-coil for signal
detection. FUS was applied with a 1-MHz 256-element phased-array transducer
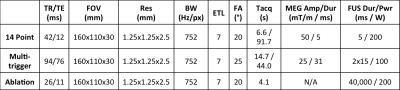
(Imasonic/IGT, France). MR and FUS parameters are listed in Table 1. To
investigate changes in tissue stiffness with ablation a 14 MP-MR-ARFI
acquisition (13 FUS-ON acquisitions interleaved with one FUS-OFF acquisition) was
performed in an ex-vivo bovine brain. After tissue ablation at the geometric
focus (40s/200W continuous sonication) the same 14 MP-MR-ARFI protocol was repeated.
The 13 FUS-ON images from the initial MP-MR-ARFI were retrospectively down
sampled by removing various numbers of ky- and/or kz-lines to investigate the effect
of view sharing reconstruction on displacement maps. A second MP-MR-ARFI
experiment where 2 FUS-ON acquisitions were interleaved with one FUS-OFF
acquisition, but each FUS-ON acquisitions had the FUS sequentially focusing in
2 positions 5-mm apart, were also performed in a porcine brain.
Results
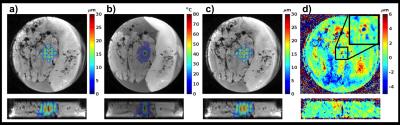
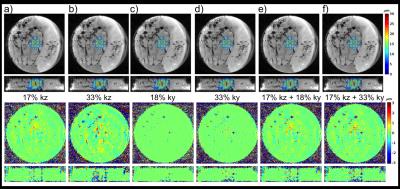
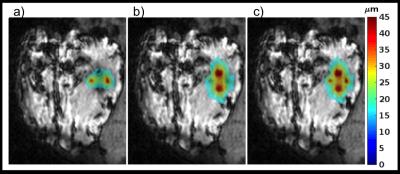
Figure 1 shows displacement maps before and after tissue ablation, as well as a difference map. A 22% post-ablation decrease in displacement was observed at location where maximum ablation temperature was achieved. Figure 2 shows displacement maps and error maps for view sharing reconstruction of 17% and 33% in kz, 18 and 33% in ky, and combination of 17% in kz with 18 and 33% in ky. The six different amounts would reduce Tacq from 91.7 s to between 77.5 and 54.1 s. Figure 3 shows individual and MIP displacement maps for the multi-trigger experiment.Discussion and Conclusion
This work has shown that the stiffness of ex-vivo brain tissue changes with ablation, but in-vivo validation is needed. The reconstructed displacement maps are relatively robust to view sharing in ky, and combining with limited kz-view sharing scan time reductions of 30-40% is achievable. Current hardware limitations limited the number of FUS pulses that could be emitted during a single MEG lobe without too great T2*-decay. Solving this issue, up to 6 5-ms pulses (i.e. 6 spatial locations) could be encoded during one 30 ms MEG-lobe. Combining these methods full 3D MP-displacement maps could be achieved in a clinically acceptable time of 30-60 s.Acknowledgements
This work was supported by The FUS Foundation, a University of Utah seed grant, and NIH grants R01s EB013433, and CA172787.References
1. McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K. MRI Investigation of the Threshold for Thermally Induced Blood-Brain Barrier Disruption and Brain Tissue Damage in the Rabbit Brain. Magn. Reson. Med. 2004;51:913–923. 2. Paeng D-G, Xu Z, Snell J, Quigg AH, Eames M, Jin C, Everstine AC, Sheehan JP, Lopes BS, Kassell N. Thermal dose effects by MR- guided focused ultrasound on the pig brain tissue - preliminary result. In: International Symposium on Therapeutic Ultrasound. Tel Aviv; 2016. p. 61. 3. de Bever J, Odeen H, Parker D. Simultaneous Acquisition of Acoustic Radiation Force Imaging and Proton Resonance Frequency Shift Thermometry Using Interleaved Acquisition with Temporally Constrained Reconstruction for Increased Temporal Resolution. In: Proceedings of International Society for Magnetic Resonance in Medicine. Singapore; 2016. p. 2508. 4. Parker D, de Bever J, Odeen H, Payne A, Christensen D. Tissue Stiffness Imaging with Interleaved Multiple-Point MR-ARFI. In: Focused Ultrasound Surgery Foundation 5th International Symposium. Bethesda, Md; 2016. p. BR-20.Figures