1163
Hybrid MR-PET of Brain Tumours with amino acid-PET and Amide Proton Transfer MRI1Institute of Neuroscience and Medicine - 4, Forschungszentrum Jülich, Jülich, Germany, 2Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 3Department of Brain Repair and Rehabilitation, UCL Institute of Neurology, London, United Kingdom, 4German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 5Jülich Aachen Research Alliance (JARA), Section JARA Brain, Aachen, Germany, 6Department of Nuclear Medicine, RWTH University of Aachen, Aachen, Germany, 7Department of Neurology, Faculty of Medicine, RWTH Aachen University, JARA, Aachen, Germany, 8Department of Electrical and Computer Systems Engineering, and Monash Biomedical Imaging, School of Psychological Sciences, Monash University, Melbourne, Australia
Synopsis
Information on amino acid metabolism has become an important component of the diagnostic and prognostic precision in the investigation of brain tumours. Both MRI and PET present methods to obtain amino acid weighted images, such as chemical exchange saturation transfer (CEST) or O-(2-18F- fluoroethyl)-L-tyrosine (FET), respectively. In this work, FET-PET and CEST imaging of brain tumours is investigated using hybrid MR-PET in 8 patients with brain tumours. The results suggest that the tumour-to-brain ratio of magnetization transfer ratio asymmetry (MTRasym) encodes different information than that from FET PET.
Purpose
Information on amino acid metabolism has become an important component of the diagnostic and prognostic precision in the investigation of brain tumours. On the one hand, PET using radiolabelled amino acids such as O-(2-18F- fluoroethyl)-L-tyrosine (FET) is a powerful tool for defining the volume of brain tumours for treatment planning, treatment monitoring and recurrence evaluation1. On the other hand, CEST at 3.5 ppm or amide proton transfer (APT) has also been demonstrated to provide information about tumour grading and tumour recurrence2, 3. Comparative brain tumour imaging with both methods, however, has not yet been investigated in the literature. The present work investigates and presents a methodology to obtain volumetric APT-CEST and FET PET to study brain tumours using simultaneous MR-PET measurements at 3T.Methods
Data from eight tumour patients were included in this study. All subjects presented FET positive areas. MR acquisitions were performed on a 3T MR-BrainPET scanner (MAGNETOM Trio, Siemens Healthineers, Erlangen, Germany). CEST imaging was included in our clinical protocol, in addition to the standard FLAIR and MPRAGE pre/post contrast acquisitions. CEST acquisition was based on a steady-state approach4. A 3D segmented EPI sequence using 3 shots per partition was used. A 100ms Gaussian pulse was followed by a delay of 36ms after which a crusher gradient was applied and readout was then performed. In order to avoid contributions from fat signals, a square excitation pulse of 2.4ms duration was used5. A B1 average power of 1μT was used. Image readout details were as follows: TE=12.5ms, readout duration=26ms, matrix size=80×80×48, voxel size=3mm3 and single image acquisition in 18 seconds. The Z-spectrum was unevenly sampled at 69 different frequencies between 14 to -14ppm and at 300ppm for data normalization. Water saturation shift referencing (WASSR) data were also acquired using the same readout as the CEST measurement6. Simultaneously with the MR measurements, an FET PET scan was carried out. PET data were reconstructed using OP-OSEM3D software with 4 subsets and 32 iterations and the images were normalized and corrected for attenuation, scatter, dead time and random7, resulting in images with voxels of 1.25×1.25×1.25mm3 and matrix size of 256×256×153. After data acquisition, field inhomogeneities were corrected using WASSR. APT-weighted images were calculated with magnetization transfer ratio asymmetry at 3.5ppm:
MTRasym = Z(@3.5ppm) – Z(@-3.5ppm),
where Z is the normalised Z-spectrum with the data acquired at 300 ppm.
A volume-of-interest (VOI) analysis was performed to compare the MTRasym and the FET data. A VOI was defined including normal-appearing white matter based on the MPRAGE and FLAIR data. Tumour VOI was defined based on the metabolically active area depicted in FET PET as a clinical reference8. Statistical analysis of the tumour-to-brain ration (TBR) of the CEST data was performed against PET data using the non-parametric Wilcoxon test. A statistical significance threshold of p<0.05 was considered using PASW Statistics software (Release 22.0.0, SPSS Inc., Chicago, IL). All the processing was performed using developed in-house MATLAB (The MathWorks Inc., Natick, MA) scripts.
Results and Discussion
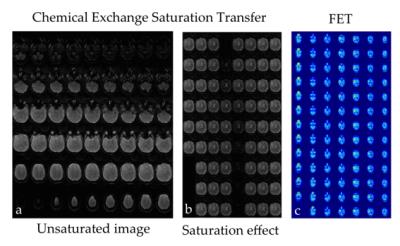
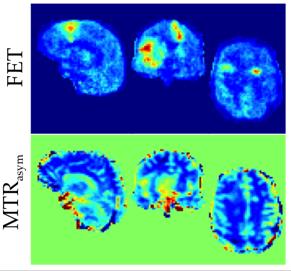
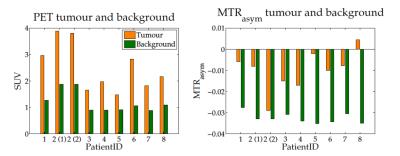
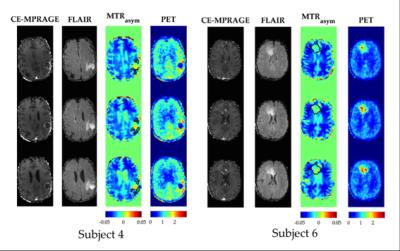
The proposed methodology allows one to obtain full brain hybrid amino acid weighted imaging within a clinically feasible scan time (fig. 1). Full brain coverage can be clinically valuable as demonstrated by fig. 2, which shows that more than one active tumour area can be present. The MTRasym for all the FET positive areas and normal apparent white matter were -0.010±0.009 and -0.031 ±0.003, respectively. Data for each subject is presented in fig. 3 and parametric images in fig. 4. Those are in agreement with the literature where at 1μT NAWM presents reduced signal than tumour areas10. When comparing the TBR of MTRasym and FET, the null hypothesis could not be rejected (p>0.05), suggesting that the two modalities encode different information in terms of TBR. MTRasym is the CEST metric often used at 3T. However, both APT and NOE influence this CEST metric and it is not a direct measurement of amino acids. In addition, the higher powers often used introduce a higher dependency on the macromolecular pool9,10. Consequently, the use of a method that considers the macromolecular pool and disentangles NOE and APT should be investigated11, 12. In addition, PET with different amino-acids can also be considered13, 14.Conclusions
For the first time CEST imaging was compared with FET in a simultaneous MR-PET measurement. The initial results suggest that MTRasym TBR encodes different information in the metabolically active area when compared to FET PET TBR.Acknowledgements
No acknowledgement found.References
1. Galldiks N and Langen K-J (2016) Amino Acid PET – An Imaging Option to Identify Treatment Response, Post-therapeutic Effects, and Tumor Recurrence? Front Neurol. 7:120
2. Sakata A, Okada T, Yamamoto A et al. (2015) Grading glial tumors with amide proton transfer MR imaging: different analytical approaches. J. Neurooncol 122(2):339-48
3. Wen Z, Hu S, Huang F et al. (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 51(2):616-22
4. Jones CK, Polders D, Hua J et al. (2012) In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn Reson Med 67(6):1579-89
5. Stirnber R, Brenner D, Stöcker T et al. (2016) Rapid fat suppression for three-dimensional echo planar imaging with minimized specific absorption rate. Magn Reson Med 76(5):1517-1523
6. Kim M, Gillen J, Landman BA et al. (2009) Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med 61(6):1441-1450
7. Herzog H, Langen KJ, Weirich C, et al. (2011) High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin. 50(2):74-82.
8. Pauleit D, Floeth F, Hamacher K, et al. (2005) O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 128:678-687.
9. Desmond KL and Stanisz GJ (2012) Understanding quantitative pulsed CEST in the presence of MT. Magn Reson Med 67(4):979-90
10. Zhao X, Wen Z, Huang F et al. (2011) Saturation power dependence of amide proton transfer image contrasts in human brain tumors and strokes at 3 T. Magn Reson Med 66(4):1033-41
11 Heo HY, Zhang Y, Jiang S et al. (2016) Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semisolid magnetization transfer reference (EMR) signals: II. Comparison of three EMR models and application to human brain glioma at 3 Tesla. Magn Reson Med 75(4):1630-9
12. Zaiss M, Windschuh J, Paech D et al. (2015) Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage 112:180-8.
13. Harris RJ, Cloughesy TF, Liau LM et al. (2015) pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neuro Oncol 17(11):1514-24
14. Lapa C, Linsenmann T, Monoranu CM et al. (2014) Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med 55(10):1611-6.
Figures