1159
GPR88 signatures on the reward resting state brain networks after alcohol exposure in mice1Advanced Molecular Imaging Center, Medical Physics, Department of Radiology, University Medical Center Freiburg, Freiburg, Germany, 2Faculty of Biology, University of Freiburg, Freiburg, Germany, 3Department of Translational Medicine and Neurogenetics, Institute of Genetics and Molecular and Cellular Biology (IGBMC), Illkirch-Graffenstaden, Strasbourg, France, 4Department of Psychiatry, Douglas Hospital Research Center, School of Medicine, McGill University, Montreal, QC, Canada, 5Spemann Graduate School of Biology and Medicine, University of Freiburg, Germany, 6Department of Biophysics and Nuclear Medicine, University Hospital Strasbourg, Strasbourg, France, 7Laboratory of Engineering, Informatics and Imaging, University of Strasbourg, Strasbourg, France
Synopsis
Identifying the genetic and molecular factors regulating the development and the dynamics of brain functional connectivity (FC) networks in health and disease is important to develop novel therapeutic strategies. Resting state functional magnetic resonance imaging (rsfMRI) can reveal the FC remodeling in psychiatric disorders and drug addiction. Emerging studies suggest that neuronal responses to alcohol involve several G protein-coupled receptors (GPCR)-mediated signaling pathways, inducing short-term to long-term changes in behavioral and neuronal plasticity. This study investigates the role of GPR88 in the acquisition and development of alcohol dependence and unravels the rsFC modifications underpinning this processes in the mouse brain.
Introduction
Alcohol is the most widely used addictive substance worldwide affecting the resting state functional connectivity (rsFC) of the brain1-3. Chronic addictive drug use is often related to abnormal brain functional organization leading habitually hypersensitivity to the drug and ensures compulsive drug-seeking behavior4. GPR88 is an orphan GPCR robustly expressed in striatal dopamine D1-receptor (D1R) and D2R expressing medium spiny neurons5, as well as in the central amygdala (CEA)6. Brain mesolimbic dopamine (DA) systems play key roles in natural rewards and in the development of drug dependence7,8. Emerging studies suggest that GPR88 deletion profoundly alters the DA system9-11. Moreover, modulated expression of GPR88 has been reported following antidepressants treatments or addictive drugs including alcohol12-14. However, the neuropharmacological/neuroadaptive mechanisms of GPR88 in alcohol addiction are still unknown. Therefore, this study was aimed to investigate whether GPR88 deletion remodels the reward network and mediates vulnerability to alcohol drinking behavior in mice.Methods
Animals: GPR88-/- mice (74.9% C57B/6J, 25% 129/SvPas, 0.05% FVB/N, 0.05% SJL/J) – lacking the GPR88 functional receptor were produced15. As controls, we used GPR88+/+ mice normally expressing the GPR88 receptor. 8–10 weeks old, adult male mice were housed with water and alcohol respectively (GPR88+/+ water:N=10, GPR88-/- water:N=10, GPR88+/+ alcohol:N=10 and GPR88-/- alcohol:N=10).
Alcohol administration and behavioral experiment: The level of voluntary alcohol intake was measured in the home cage, using 20% alcohol intermittent two bottle-choice drinking paradigm. Mice (single housed under 12h reversed light/dark cycle) were given 24h of concurrent access to one bottle of 20% alcohol (v/v) and bottle of water on Monday, Wednesday and Friday with 24h/48h of alcohol-deprivation periods between the alcohol-drinking sessions. Bottles were weighed at the end of each session. The position (left/right) of each solution was alternated between sessions as a control for side preference. The possible loss of solutions because of handling was controlled by weighting bottles in empty cages. Mice were exposed to alcohol for 2 months and scanned 24h after the last alcohol drinking session.
MRI experiment: Mouse physiological conditions were monitored and stabilized during imaging performed under medetomidine (MD) sedation (subcutaneous (s.c.) bolus of 0.3mg MD/kg body weight (BW) followed by s.c. infusion of 0.6mg MD/kg-BW/hour). MRI was carried-out using a 7T small bore animal scanner (Biospec 70/20, Bruker, Germany) and a mouse head adapted cryocoil. rsfMRI data was acquired using single shot GE-EPI (TE/TR=10ms/1700ms), 12 axial slices of 0.7mm thickness (FOV:19.2×12mm², resolution:0.15×0.15mm2, 200 volumes).
Data analysis: Behavioral data regarding the alcohol intake were analyzed using two-way ANOVA with repeated measures (RM) or t-test statistics (GraphPad Prism). Significant main effects and interactions of the ANOVAs were investigated with the method of contrast analysis (p<0.05). rsfMRI data was pre-processed using SPM8 for motion correction, spatial normalization and alignment with a study based template as well as smoothing (0.4×0.4×1mm³). Using pre-processed rsfMRI data, here we exemplify the rsFC patterns of ventral tegmental area (VTA) and central amygdala (CEA), well known core players of the reward processing. Correlation coefficients were computed between the ROI and averaged time series of the remaining whole brain and converted to z-values using Fisher’s r-to-z transformation.
Results and Discussion
Deletion of GPR88 in mice increased daily alcohol consumption (Fig.1A), however did not affect water intake compared to GPR88+/+ mice (data not shown). Mean daily alcohol consumption was significantly increased in GPR88-/- mice by 34% (p<0.001) (Fig.1B). Two-way RM ANOVA showed significant main effects of Genotype (F(1,23)=20.7,p<0.001) and Sessions (F(19,437)=1.7,p<0.05) but no significant interactions between Genotype x Session (F(19,437)=0.6,p=0.9). Seed analysis quantified strong VTA (Fig.2) and CEA (Fig.3) rsFC modifications due to alcohol consumption in both GPR88+/+ and GPR88-/- groups, predominantly toward the orbito-frontal cortex, striatum, amygdala, thalamus and midbrain area (p<0.001). VTA as a central hub of the brain reward network projects DA neurons to several regions potentially involved in addiction, such as, prefrontal cortex16, nucleus accumbens17, and CEA18. Activity of DA neurons is influenced by novel stimuli and responds to unexpected natural rewards and conditioned cues that predict reward19-21. Plasticity in this system is strongly implicated in addictive disorders that involve compulsive drug-seeking22,23. Extensive modifications in VTA and CEA rsFC might be part of the reward related pathways underlying the susceptibility to alcohol intake behavior observed in the GPR88-/- mice.Conclusion
GPR88-/- mice showed vulnerability to alcohol intake and modified rsFC patterns of brain regions associated with the reward circuitry. These results support further investigations of the rsFC of other brain regions controlling reward related behavior along with the brain micro-structural organization, to expand our knowledge on the brain adaptations to alcohol exposure as well as the implications of GPR88 receptor in alcoholism.Acknowledgements
This study was supported by the Earsmus Mundus Joint Doctoral (EMJD) fellowship - “NeuroTime” program.References
1. Khalili-Mahani N, Zoethout RM, Beckmann CF, Baerends E, de Kam ML, Soeter RP et al. Effects of morphine and alcohol on functional brain connectivity during "resting state": a placebo-controlled crossover study in healthy young men. Hum Brain Mapp 2012; 33: 1003 – 1018.
2. Weber AM, Soreni N, Noseworthy MD. A preliminary study on the effects of acute ethanol ingestion on default mode network and temporal fractal properties of the brain. MAGMA 2014; 27: 291 – 391.
3. Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, Schulte T. The resting brain of alcoholics. Cereb Cortex 2014; 25: 4155 – 4168.
4. Kalivas PW and Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005; 162(8): 1403-13.
5. Massart, R., Guilloux, J.-P., Mignon, V., Sokoloff, P., Diaz, J. Striatal GPR88 expression is confined to the whole projection neuron population and is regulated by dopaminergic and glutamatergic afferents. Eur. J. Neurosci 2009; 30: 397–414.
6. Massart R, Mignon V, Stanic J et al., Developmental and adult expression patterns of the G-protein-coupled receptor GPR88 in the rat: Establishment of a dual nuclear-cytoplasmic localization. J Comp Neurol 2016; 524 (14): 2776 – 802.
7. Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2001; 2: 695-703.
8. Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001; 24:97-129.
9. Logue, S.F., Grauer, S.M., Paulsen, J., et al., The orphan GPCR, GPR88, modulates function of the striatal dopamine system: a possible therapeutic target for psychiatric disorders? Mol. Cell. Neurosci 2009; 42, 438–447.
10. Quintana, A., Sanz, E., Wang, et al., Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat. Neurosci 2012; 15: 1547–1555.
11. Ingallinesi, M., Le Bouil, L., Biguet, N.F., et al., Local inactivation of Gpr88 in the nucleus accumbens attenuates behavioral deficits elicited by the neonatal administration of phencyclidine in rats. Mol. Psychiatry 2015; 20: 951–958.
12. Befort, K., Filliol, D., Ghate, A., Darcq, E., Matifas, A., Muller, J., Lardenois, A., Thibault, C., Dembele, D., Le Merrer, J., Becker, J. a. J., Poch, O., Kieffer, B.L. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur. J. Neurosci 2008; 27: 2973–2984.
13. Conti, B., Maier, R., Barr, A.M., Morale, M.C., Lu, X., Sanna, P.P., Bilbe, G., Hoyer, D., Bartfai, T. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol. Psychiatry 2006; 12: 167–189.
14. Le Merrer J, Befort K, Gardon O et al., Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol 2012; 17: 1-12.
15. Meirsman, A.C., Le Merrer, J., Pellissier, L.P., et al.,. Mice Lacking GPR88 Show Motor Deficit, Improved Spatial Learning, and Low Anxiety Reversed by Delta Opioid Antagonist. Biol. Psychiatry 2016; 79: 917–927.
16. Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci 2007; 27(8): 2045 – 57.
17. Beitner-Johnson D, Guitart X, Nestler EJ. Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. J Neurosci 1992; 12(6): 2165 – 76.
18. Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res 1998; 784(1-2): 105 -15.
19. Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 2000; 96: 651–656.
20. Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev 2007; 56: 27–78.
21. Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004; 5: 483–494.
22. Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology 2004; 47(Suppl 1): 61–79
23. Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron 2008; 59: 486–496.
Figures
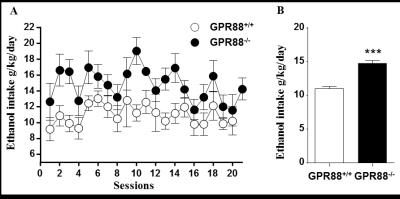
Increased alcohol consumption in mice lacking GPR88 receptor:
A. GPR88-/- mice consumed more alcohol than GPR88+/+ mice during 20% alcohol intermittent two bottle-choice drinking paradigm. Data represents the mean (±SEM) of alcohol consumption per session.
B. The mean daily alcohol consumption by GPR88+/+ and GPR88-/- mice. Data represents mean (±SEM) of daily alcohol consumption during the experiment. ***P < 0.001 compared with GPR88+/+ group.
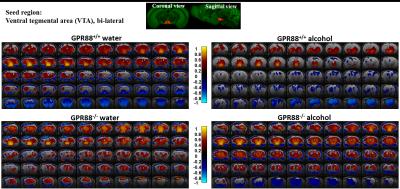
Alcohol consumption modifies the VTA connectivity in GPR88+/+ and GPR88-/- groups:
Seed region (bi-lateral VTA, extracted from Allen mouse brain atlas) is shown in coronal and sagittal plane. BOLD rsfMRI correlation maps (p<0.001) of the (from top left to bottom right) GPR88+/+ water, GPR88+/+ alcohol, GPR88-/- water and GPR88-/- alcohol group were over-laid on a T2-weighted
anatomical brain slices. The color scale indicates the T-value (positive
correlations from 0 to +1: dark red to yellow and negative correlations
from 0 to -1: dark blue to turquoise).
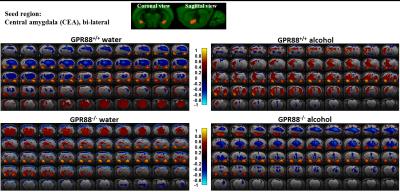
Modified CEA connectivity due to alcohol intake in GPR88+/+ and GPR88-/- groups:
Seed region (bi-lateral CEA, extracted from Allen mouse brain atlas) is shown in coronal and sagittal plane. BOLD rsfMRI correlation maps (p<0.001) of the (from top left to bottom right) GPR88+/+ water, GPR88+/+ alcohol, GPR88-/- water and GPR88-/- alcohol group were over-laid on a T2-weighted anatomical brain slices. The color scale indicates the T-value (positive correlations from 0 to +1: dark red to yellow and negative correlations from 0 to -1: dark blue to turquoise).