1156
Low frequency hippocampal-cortical activity contributes to brain-wide connectivity as measured by resting-state fMRI1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, Hong Kong, 2Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
The hippocampus, including dorsal dentate gyrus (dDG), and cortex engage in bidirectional communication. We propose that low frequency activities in hippocampal-cortical pathway underlie brain-wide resting-state fMRI (rsfMRI) connectivity to mediate distinct cognitive functions and integrate sensory information. Using optogenetics and fMRI, we determined large-scale spatiotemporal specific hippocampal-cortical activity. Low, but not high, frequency optogenetic stimulation of dDG excitatory neurons evoked robust cortical and subcortical responses, and enhanced interhemispheric hippocampal and cortical rsfMRI connectivity. In addition, pharmacological inactivation of dDG decreased rsfMRI connectivity. These findings directly indicate that low frequency activity propagates in hippocampal-cortical pathway and contributes to brain-wide rsfMRI connectivity.
Purpose
The hippocampus (HP) plays a prominent role in central nervous system functions, particularly in episodic memory1,2 and spatial navigation3,4. The HP, including dentate gyrus (DG), can evoke large-scale influences on cortical activity via cortico-hippocampal-cortical network. Specifically, dorsal HP (dHP) can integrate multimodal sensory information and process memory operations5 using excitatory long-range projections6. Resting-state fMRI (rsfMRI)7-10 maps long-range brain-wide functional connectivity networks based on temporal coherence of infra-slow (0.005-0.1Hz) BOLD activity. Previous attempts uncovering the neural basis of rsfMRI implicated delta11,12 and alpha13,14 oscillations, with cortical slow oscillations15,16 resembling infra-slow activity in rsfMRI networks17-19. However, the answer remains inconclusive due to the correlative nature of those studies without directly probing the effects of modulating brain’s activity during rsfMRI. Hippocampus has been identified as a densely connected region that integrates and distributes information within and among neural networks to coordinate brain functional connectivity20,21. These studies suggest that controlling neuronal activities in HP and examining cortical rsfMRI connectivity may present a pivotal approach to uncovering the role of HP in rsfMRI. Currently, there is no direct evidence that demonstrates the initiation and propagation of low frequency activity in HP along the hippocampal-cortical axis or its influences on rsfMRI connectivity. Since DG primarily receives cortical terminal projections and acts as a bridge for cortico-hippocampal-cortical network communication, we examined how low frequency or high frequency activities optogenetically initiated in dorsal DG (dDG), as well as the pharmacological inactivation of dDG, influence the brain-wide cortical activity and modulate the rsfMRI connectivity.Materials and Methods
Adult male Sprague-Dawley rats underwent optogenetic fMRI (n=6, 1Hz and 40Hz stimulation), and rsfMRI (n=18, low frequency stimulation; n=8, high frequency stimulation; n=12, pharmacological inactivation using 5-10ng/μL of tetrodotoxin). fMRI and rsfMRI data were acquired at 7T using single-shot Gradient-Echo Echo-Planar-Imaging sequence with FOV=32×32mm2, matrix=64×64, flip-angle=56° (optogenetic fMRI) or 50° (rsfMRI), TE=20ms, TR=1000ms (optogenetic fMRI) or 750ms (rsfMRI). Subsequently, standard preprocessing followed by GLM and seed-based analyses were applied to fMRI and rsfMRI data, respectively.Results
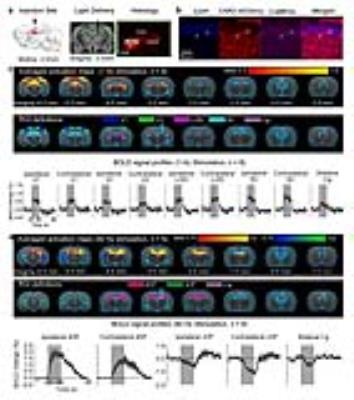
Anatomical MRI scans confirmed the location of virus injection and fiber implantation in dDG (Fig. 1a). Histology and immunohistochemistry confirmed CaMKIIα-positive excitatory neurons of dDG expressed ChR2-mCherry (Fig. 1a-b). Multi-unit activity (MUA) recordings in dDG showed 1Hz and 40Hz stimulation successfully elicited hippocampal spikes without failure (data not shown). 1Hz stimulation of dDG evoked positive and robust BOLD responses in bilateral primary visual cortex (V1), secondary visual cortex (V2), lateral geniculate nucleus (LGN) and superior colliculus (SC), as well as cingulate cortex (Cg; Fig. 1c). We also stimulated dDG at a higher frequency (40Hz) and observed strong positive responses in bilateral dHP, and negative responses in bilateral ventral HP (vHP) and Cg (Fig. 1d). Note that no evoked responses were observed in the naïve animal (data not shown), indicating that the observed responses were a direct result of ChR2 stimulation, not heating induced artifacts or undesired light-induced activations of the visual pathway. Also note that optic fibers were made opaque during all MRI experiments to prevent undesired direct visual stimulation.
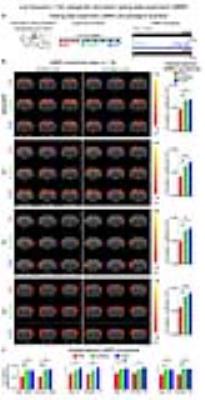
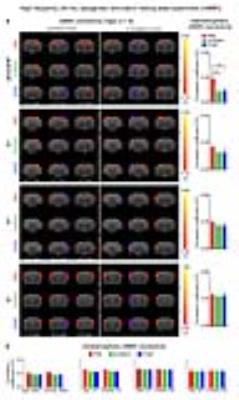
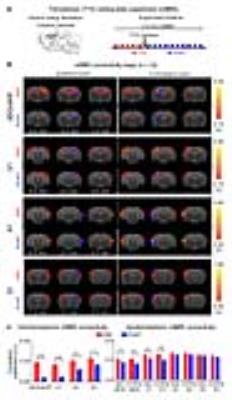
The interhemispheric rsfMRI connectivity increased in dHP, V1, primary auditory cortex (A1) and primary somatosensory cortex (S1) during (DURING) and after (POST) 1Hz dDG stimulation, which exhibited as an increase in the spatial extent of connectivity maps (Fig. 2b). Interhemispheric rsfMRI connectivity strengthened significantly DURING and POST in dHP, V1, A1 and S1 (Fig. 2b). Additionally, the intrahemispheric rsfMRI connectivity increased in dHP, V1, A1 and S1 DURING and POST stimulation (Fig. 2c). In contrast, no significant increase in inter- and intrahemispheric rsfMRI connectivity was observed DURING and POST 40Hz optogenetic stimulation of dDG (Fig. 3). Only interhemispheric rsfMRI connectivity in dHP exhibited a significant decrease in strength. On the other hand, TTX pharmacological blockade of dDG activities significantly decreased rsfMRI interhemispheric connectivity in dHP, V1, A1 and S1, and intrahemispheric connectivity in dHP and V1 (Fig. 4c-d).
Discussion and Conclusion
The frequency specific fMRI responses (Fig. 1c-d) demonstrates that spatiotemporal response properties from dHP transitions activity from regions actively involved in processing visual information and cognition at low frequency (1Hz) to local hippocampal regions (40Hz). More importantly, low frequency dDG stimulation enhanced interhemispheric hippocampal and cortical rsfMRI connectivity (Fig. 2), but not high frequency stimulation (Fig. 3). These results directly indicate that persistent low, but not high, frequency activity propagating from dDG along the hippocampal-cortical pathway enhances interhemispheric rsfMRI connectivity. In addition, pharmacological inactivation of dDG/dHP decreased rsfMRI connectivity (Fig. 4). Together, these results directly reveal that low frequency activity propagates along the hippocampal-cortical pathway and plays a pivotal role in driving the brain-wide interhemispheric cortical connectivity as measured by rsfMRI.Acknowledgements
No acknowledgement found.References
1. Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry 20, 11-21 (1957).
2. Squire, L. R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review 99, 195-231 (1992).
3. Maguire, E. A. et al. Knowing where and getting there: a human navigation network. Science (New York, N.Y.) 280, 921-924 (1998).
4. Buzsaki, G. & Moser, E. I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature neuroscience 16, 130-138, doi:10.1038/nn.3304 (2013).
5. Fanselow, M. S. & Dong, H. W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7-19, doi:10.1016/j.neuron.2009.11.031 (2010).
6. Buzsaki, G. The hippocampo-neocortical dialogue. Cerebral cortex (New York, N.Y. : 1991) 6, 81-92 (1996).
7. Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8, 700-711, doi:10.1038/nrn2201 (2007).
8. Smith, S. M. et al. Resting-state fMRI in the Human Connectome Project. NeuroImage 80, 144-168, doi:10.1016/j.neuroimage.2013.05.039 (2013).
9. Buckner, R. L., Krienen, F. M. & Yeo, B. T. Opportunities and limitations of intrinsic functional connectivity MRI. Nature neuroscience 16, 832-837, doi:10.1038/nn.3423 (2013).
10. Zhou, I. Y. et al. Brain resting-state functional MRI connectivity: morphological foundation and plasticity. NeuroImage 84, 1-10, doi:10.1016/j.neuroimage.2013.08.037 (2014).
11. Lu, H. et al. Low- but Not High-Frequency LFP Correlates with Spontaneous BOLD Fluctuations in Rat Whisker Barrel Cortex. Cerebral cortex (New York, N.Y. : 1991) 26, 683-694, doi:10.1093/cercor/bhu248 (2016).
12. Lu, H. et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proceedings of the National Academy of Sciences of the United States of America 104, 18265-18269, doi:10.1073/pnas.0705791104 (2007).
13. Wang, L., Saalmann, Y. B., Pinsk, M. A., Arcaro, M. J. & Kastner, S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron 76, 1010-1020, doi:10.1016/j.neuron.2012.09.033 (2012).
14. Scholvinck, M. L., Maier, A., Ye, F. Q., Duyn, J. H. & Leopold, D. A. Neural basis of global resting-state fMRI activity. Proceedings of the National Academy of Sciences of the United States of America 107, 10238-10243, doi:10.1073/pnas.0913110107 (2010).
15. Buzsaki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926-1929, doi:10.1126/science.1099745 (2004).
16. Sheroziya, M. & Timofeev, I. Global intracellular slow-wave dynamics of the thalamocortical system. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 8875-8893, doi:10.1523/jneurosci.4460-13.2014 (2014).
17. Matsui, T., Murakami, T. & Ohki, K. Transient neuronal coactivations embedded in globally propagating waves underlie resting-state functional connectivity. Proceedings of the National Academy of Sciences of the United States of America 113, 6556-6561, doi:10.1073/pnas.1521299113 (2016).
18. Amemiya, S., Takao, H., Hanaoka, S. & Ohtomo, K. Global and structured waves of rs-fMRI signal identified as putative propagation of spontaneous neural activity. NeuroImage 133, 331-340, doi:10.1016/j.neuroimage.2016.03.033 (2016).
19. Mitra, A., Snyder, A. Z., Tagliazucchi, E., Laufs, H. & Raichle, M. E. Propagated infra-slow intrinsic brain activity reorganizes across wake and slow wave sleep. eLife 4, e10781, doi:10.7554/eLife.10781 (2015).
20. Cole, M. W., Pathak, S. & Schneider, W. Identifying the brain's most globally connected regions. NeuroImage 49, 3132-3148, doi:10.1016/j.neuroimage.2009.11.001 (2010).
21. Power, J. D., Schlaggar, B. L., Lessov-Schlaggar, C. N. & Petersen, S. E. Evidence for hubs in human functional brain networks. Neuron 79, 798-813, doi:10.1016/j.neuron.2013.07.035 (2013).
Figures