1152
On- and off-target circuit effects of high frequency electrical deep brain stimulation at the subthalamic nucleus1Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2National Chiao-Tung University, 3Neurology, UNC at Chapel Hill, Chapel Hill, NC, United States
Synopsis
This study introduces a novel hybrid methodology for DBS-fMRI research. Our findings demonstrate robust stimulus evoked fMRI and fcMRI responses during STN-DBS, which should shed light on how DBS exerts its therapeutic effects on whole-brain functional networks and delineate a road-map for future optimization of DBS therapy to enhance outcomes and reduce side-effects.
Purpose
Deep brain stimulation (DBS) is a well-established neurosurgical therapy for multiple neurological and psychiatric disorders. It is most commonly employed in the treatment of Parkinson’s disease (PD) when medical therapy becomes inadequate or dyskinesias become intolerable1. When applied for the treatment of PD, the subthalamic nucleus (STN) is frequently targeted, often resulting in a marked reduction in several hallmark PD symptoms, including resting tremor, bradykinesia and rigidity. Despite these benefits, many parkinsonian symptoms are frequently refractory to, or may worsen during DBS treatment, mainly because the mechanisms by which STN-DBS exerts its therapeutic effects remain unclear1. Studying DBS effects using fMRI in human patients is difficult due to technical and safety constraints; consequently, DBS studies in small animals are drawing increasing attention. In addition to overcoming many of the technical limitations of DBS-fMRI present in humans, preclinical models allow for increased flexibility to investigate brain circuits other than established clinical targets, as well as the possibility to employ a wide variety of cutting-edge basic research tools. Unfortunately, conventional DBS-fMRI studies in small animal models, including recent studies from our own group, suffer from both DBS electrode-induced artifacts and cases of electrode mistargeting2-5. To address these issues, we developed a novel 16-channel MR-compatible vertical microelectrode array using advanced micromachining techniques6. This microelectrode array substantially increases the probability of contact lead placement within the STN along the dorso-ventral axis, where STN targeting is most difficult. We employed state-of-the-art isotropic fMRI and functional connectivity MRI (fcMRI) techniques in rats to quantitatively map the neural circuits modulated by STN-DBS at the clinically-relevant stimulation frequency of 130Hz. Additionally, we examined on-target and off-target DBS effects in a single scan-session, aiming to more accurately identify the brain regions and circuits recruited by STN-DBS.Methods
Wildtype Sprague-Dawley rats were implanted with MR-compatible 16 channel microelectrodes unilaterally to the STN guided by electrophysiology (n=5). To prepare for DBS-fMRI experiments, each rat was ventilated with 0.5% isoflurane and medical air. Intraperitoneal infusion of dexmedetomidine (0.05mg/kg/hr) and pancuronium bromide (0.5mg/kg/hr) were given for the duration of scan. CBV fMRI was achieved by injecting 20mg-Fe/kg iron oxide contrast. A single shot GE-EPI sequence (BW=150kHz, TR=2000ms, TE=11.2ms, matrix=72x72, slice=32, FOV=2.88x2.88x1.28cm, and resolution=0.4mm isotropic) was used to acquire on a Bruker 9.4T MR scanner with a volume transmitter coupled with a 4-channel array receiver. STN-DBS was conducted using 500µA, 130Hz, and 0.3ms pulse duration. A 210s block design paradigm was implemented, consisting of a 60s baseline period followed by 30s of stimulation ON, and an additional 120 seconds of rest. fcMRI scans then were conducted with scan series consisted of two, 5min scans during which either no stimulation or continuous DBS was applied (OFF and ON, respectively). Preprocessing and image analysis was performed using AFNI software suit, ANTs toolkit, and a custom-written python package. Data were first realigned to the first volume of a well-positioned subject using a least squares approach followed by a 6-parameter rigid body spatial transformation. Skull-stripping was then employed using a semi-automatic threshold method with manual masking, followed by image coregistration to an anatomical MRI rat atlas7.Results and discussion
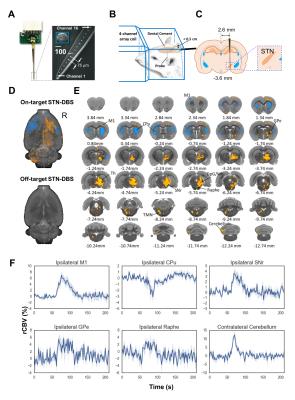
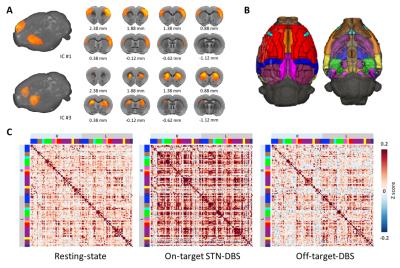
With electrophysiology-guided implantation and the use of the MR-compatible microelectrode array (Figure 1A-C), we observed robust STN-DBS evoked-fMRI signal increases in the ipsilateral primary motor cortex (M1), external globus pallidus (GPe), motor thalamus (VA/VL), substantia nigra pars reticulata (SNr), deep gray layer and white layer superior colliculus (DpG/Wh), raphe, contralateral cerebellum, and trigeminal motor nucleus (TMN). fMRI signal decreases were also observed in bilateral dorsal striatum (CPu) and contralateral M1 (Figure 1D-F). Most of these changes were not observed in our previous STN-DBS study5, which we attribute to improved DBS accuracy achieved by the microelectrode array, electrophysiology-guided targeting, and refined high resolution isotropic imaging protocols described herein. Interestingly, off-target STN-DBS at 0.3 mm above the stimulation target did not result in any detectable response in all the subjects, possibly due to depolarization blocks under our experimental conditions. During fcMRI experiments, robust circuit connectivity patterns were observed at rest (Figure 2A), while distinct network patterns emerged during on-target and off-target STN-DBS (Figure 2C). Most notably, on-target STN-DBS significantly strengthened connectivity among multiple brain networks, with a few exceptions including the parietal cortices within the default mode network and the visual network.Conclusion
This study introduces a novel hybrid methodology for DBS research. Our findings should shed light on how DBS exerts its therapeutic effects on whole-brain functional networks and delineate a road-map for future optimization of DBS therapy to enhance outcomes and reduce side-effects.Acknowledgements
We thank members of the Shih lab for valuable discussions concerning the experiments described in this manuscript. The Shih lab is supported by NINDS R01 NS091236, NIMH R01 MH111429, R41 MH113252, R21 MH106939, NIAAA U01 AA020023, R01 AA025582, American Heart Association 15SDG23260025, and Brain & Behavior Research Foundation.References
1
Albaugh, D. L. & Shih, Y. Y. Neural circuit modulation during deep brain
stimulation at the subthalamic nucleus for Parkinson's disease: what have we
learned from neuroimaging studies? Brain Connect 4, 1-14,
doi:10.1089/brain.2013.0193 (2014).
2
Albaugh, D. L. et al. Functional Magnetic Resonance Imaging of
Electrical and Optogenetic Deep Brain Stimulation at the Rat Nucleus Accumbens.
Sci Rep 6, 31613, doi:10.1038/srep31613 (2016).
3
Canals, S., Beyerlein, M., Murayama, Y. & Logothetis, N. K. Electric
stimulation fMRI of the perforant pathway to the rat hippocampus. Magn Reson
Imaging 26, 978-986, doi:10.1016/j.mri.2008.02.018 (2008).
4
Krautwald, K., Min, H. K., Lee, K. H. & Angenstein, F. Synchronized
electrical stimulation of the rat medial forebrain bundle and perforant pathway
generates an additive BOLD response in the nucleus accumbens and prefrontal
cortex. Neuroimage 77, 14-25,
doi:10.1016/j.neuroimage.2013.03.046 (2013).
5
Lai, H. Y., Younce, J. R., Albaugh, D. L., Kao, Y. C. & Shih, Y. Y.
Functional MRI reveals frequency-dependent responses during deep brain
stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage
84, 11-18, doi:10.1016/j.neuroimage.2013.08.026 (2014).
6
Lai, H. Y. et al. Design, simulation and experimental validation of a
novel flexible neural probe for deep brain stimulation and multichannel
recording. J Neural Eng 9, 036001,
doi:10.1088/1741-2560/9/3/036001 (2012).
7
Valdes-Hernandez, P. A. et al. An in vivo MRI Template Set for
Morphometry, Tissue Segmentation, and fMRI Localization in Rats. Front
Neuroinform 5, 26, doi:10.3389/fninf.2011.00026 (2011).
Figures