1148
Intratissue Strains Increase in a Full Thickness and Critical Sized Ovine Cartilage Defect Model1Rensselaer Polytechnic Institute, Troy, NY, United States, 2Purdue University, 3KU Leuven, 4University of Colorado Boulder, Boulder, CO, United States
Synopsis
Functional imaging of intratissue strain in articular cartilage provides an opportunity to probe the healthy or diseased state of the tissue. We utilized displacement encoded MRI to document the increase in deformation following creation of a critical sized femoral defect in a large animal model ex vivo. Strain heterogeneity indicated that the implant replacement is critical to long-term repair success and restoration of physiological cartilage strain and mechanical function. This study represents a crucial step toward the evaluation of biomechanical imaging biomarkers to evaluate tissue damage, repair, and regeneration in the intact joint of a clinically-relevant and translational animal model.
Purpose
Articular cartilage and surrounding soft tissues in the knee
are important to normal joint function. However, trauma and progressive
degeneration can diminish joint function, alter distributions of mechanical
deformation in tissues [1],
and lead to advanced osteoarthritis [2].
The ability to restore strain distributions of damaged regions to accepted
normal values could be used as a measure of success of a tissue engineering
solution [3].
MRI has shown promise in the study of soft tissue biomechanics, including displacement-encoded
MRI to directly measure mechanical behavior [4, 5].
Because displacement-encoded MRI is noninvasive, it may be applied to
longitudinal studies of cartilage treatments, which are typically performed in
animal models, including sheep. Our aim was to visualize the change in
cartilage strain during tibiofemoral contact as a result of a full-thickness
cartilage defect.Methods
Ovine stifle (knee) specimen were obtained from a local
abattoir and frozen immediately after slaughter. Prior to imaging, excess tissues
were removed, and joints were secured to a custom MRI-compatible loading device
in a 50° flexed position to replicate the stance phase of gait [6].
Gauze wet with phosphate-buffered saline (PBS) and plastic wrap was used to
prevent desiccation of the joint. Imaging was conducted on a 9.4T imaging
system (Bruker). After preconditioning [4],
MRI acquisitions were synchronized with cyclic compression of the joint.
For imaging, displacement encoding with stimulated echoes
(DENSE, [7])
and a TrueFISP acquisition (DENSE-FISP) was implemented using an encoding
gradient moment of 0.65π/mm and a mixing time of 600ms [8].
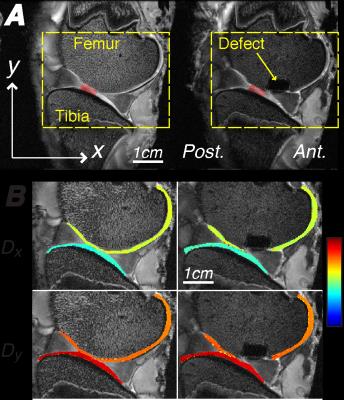
Encoding gradients were applied in the loading/transverse (y/x) directions, as previously described, to measure displacements
in the sagittal imaging plane, which was selected to cut through the most
distal aspect of the medial condyle. DENSE-FISP parameters: flip angle=25°, echo/repetition
time=1.65/3.30ms, FOV=64.0x64.0mm2,
spatial resolution=250x250µm2,
slice thickness=1.5mm, NA=8, phase advances=60°/180°/300° [8].
Prior to strain calculations, a locally-weighted scatterplot smoothing method
was applied to displacements within the cartilage using customized software
(MATLAB). Upon completion of the first set of scans, joints were
removed for surgery. An 8-mm diameter full-thickness (i.e. 5-mm deep) defect
was created at the most distal aspect of the medial femoral condyle without
disrupting the meniscus. The defect joint was again wrapped in PBS-soaked gauze
and plastic before imaging with DENSE-FISP. Changes to strains in the articular
cartilage were quantified and compared during cartilage-cartilage contact of
the areas surrounding the defect.
Results
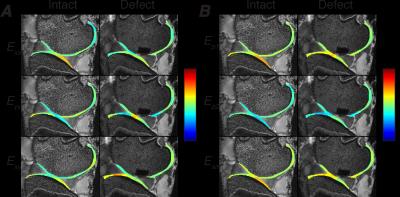
Intratissue displacements and strain were computed in the
femoral and tibial articular cartilage of the joint before and after defect
creation (Figures 1 and 2). Rigid body
displacement dominated displacements in the x
and y directions. Strain maps showed intratissue
heterogeneity which increased in femoral cartilage near the defect. In the
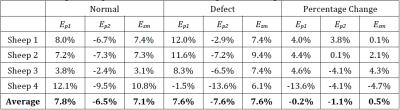
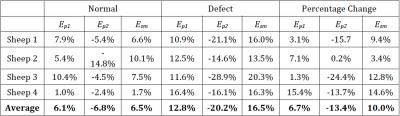
intact cartilage-cartilage contact regions, average strains increased in the
femoral, but not tibial, cartilage (Tables 1 and 2).
Discussion
The purpose of this study was to examine the changes in
cartilage displacements and strains with the introduction of a full-thickness
defect. The obtained heterogeneous strain fields represent the intratissue
deformation prior to and after defect creation. In particular, localized areas
of high tensile and shear strain were observed in the transition areas between
the cartilage-cartilage and cartilage-meniscus contact areas. With the creation
of a defect, principal strains increased only in the femoral cartilage. The
observation that tibial cartilage was not as affected by the defect may
indicate that the meniscus effectively dissipates strains to locations away
from the defect site.
The differences in the femoral strain fields after defect
creation should however be considered within the light of the following
limitations of this ex vivo study.
The defect resulted in lower signal-to-noise in surrounding areas due to
banding artifacts typical of TrueFISP sequences. Future in vivo studies are needed to evaluate the effect of cartilage
repair tissue on the mechanical environment of the surrounding cartilage.Conclusion
Our ex vivo study represents
a precursor to future in vivo studies
of defect models. The sensitivity of displacement-encoded MRI to document
increases in strain within the joint indicate progress towards establishment of
imaging-based biomechanical biomarkers for cartilage disease and repair.Acknowledgements
This research was funded in part by BioRegeneration Technologies, Inc., NSF CMMI 1100554 (CPN and EAN), NIH R01 AR063712 and R21 AR064178 (CPN), OVPR Research Incentive Grant (CPN). It was performed at the Center for Basic Magnetic Resonance Research, North Shore University HealthSystem, Evanston, IL, on equipment funded in part by NIH S10 RR019920-01.References
1. Thambyah, A. and N. Broom, On how degeneration influences load-bearing in the cartilage-bone system: a microstructural and micromechanical study. Osteoarthritis Cartilage, 2007. 15(12): p. 1410-23. 2. Guilak, F., et al., The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res, 2004(423): p. 17-26. 3. Neu, C.P., Functional imaging in OA: role of imaging in the evaluation of tissue biomechanics. Osteoarthritis Cartilage, 2014. 22(10): p. 1349-59. 4. Chan, D.D., L. Cai, K.D. Butz, S.B. Trippel, E.A. Nauman, and C.P. Neu, In vivo articular cartilage deformation: noninvasive quantification of intratissue strain during joint contact in the human knee. Sci Rep, 2016. 6: p. 19220. 5. Chan, D.D., C.P. Neu, and M.L. Hull, In situ deformation of cartilage in cyclically loaded tibiofemoral joints by displacement-encoded MRI. Osteoarthritis Cartilage, 2009. 17(11): p. 1461-8. 6. Tapper, J.E., et al., In vivo measurement of the dynamic 3-D kinematics of the ovine stifle joint. J Biomech Eng, 2004. 126(2): p. 301-5. 7. Aletras, A.H., S. Ding, R.S. Balaban, and H. Wen, DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson, 1999. 137(1): p. 247-52. 8. Chan, D.D. and C.P. Neu, Transient and microscale deformations and strains measured under exogenous loading by noninvasive magnetic resonance. PloS one, 2012. 7(3): p. e33463.
Figures