1146
Novel NMR biomarkers characterizing human knee synovial fluid after ACL-injuries: correlation with immunoassay and longitudinal cartilage MR T1ρ and T2 imaging1University of California, San Francisco, San Francisco, CA, United States, 2Duke University, Durham, NC, United States
Synopsis
Subjects with acute anterior cruciate ligament (ACL) injury have a high risk of developing post-traumatic osteoarthritis (PTOA) even after ACL reconstruction. To identify novel NMR biomarkers of synovial fluid that may predict cartilage degeneration after acute injury and to develop potential preventative strategies for PTOA, human knee synovial fluid harvested from 25 anterior cruciate ligament (ACL) injured subjects were studied using high resolution magic angle spinning (HR-MAS) NMR spectroscopy and correlated to immunoassay and longitudinal cartilage MR T1ρ and T2 imaging.
Purpose
Subjects with acute anterior cruciate ligament (ACL) injury have a high risk of developing PTOA even after ACL reconstruction. It is hypothesized that biochemical and inflammatory cascades caused by the injury may contribute to PTOA development; however, no previous studies have reported direct evidence of correlating increased inflammation after injury with longitudinal cartilage degeneration. We have previously developed high-resolution magic-angle-spinning (HR-MAS) NMR protocols and advanced reconstruction techniques to quantify metabolic concentrations and dynamics (e.g. relaxation times and diffusion coefficients) in human knee synovial fluid.[1,2] The goal of this study was to: 1) correlate synovial fluid NMR measures with immunoassay analysis to identify potential NMR biomarkers associated with joint inflammation and cartilage degeneration after ACL-injuries; 2) evaluate the capability of NMR measures to predict longitudinal cartilage degeneration measured by MR T1ρ and T2 imaging.Methods and Materials
Synovial fluid samples were collected from 25 patients (11 females, age: 33.1 ± 8.4 yr, BMI: 23.9 ± 3.5 kg/m2, days from injury to surgery: 64.9 ± 27.2) with acute ACL-injuries during ACL reconstruction, and were centrifuged and then frozen at -80ºC immediately after aspiration. The HR-MAS 1H NMR spectra were acquired on a Varian INOVA 500 MHz spectrometer at 1ºC with a consistent spin rate of 2250 kHz.[1] Concentrations and dynamics (relaxation times and diffusion coefficients) of synovial fluid metabolites were measured using optimized HR-MAS NMR protocols (saturation recovery for T1 measurement; CPMG for metabolite quantification and T2 measurement; gradient STE for D; novel reconstruction algorithm developed for 2D relaxation/diffusion spectra; good reproducibility with average CVs less than 2.5% for metabolite quantification and 7.0% for relaxometry/diffusivity).[2] Concentrations of (anti-) inflammatory cytokines and cartilage turnover markers (degradation and synthesis) were measured using immunoassays. All subjects had their knees scanned using MRI (3T GE MR750 wide bore and 8 channel knee coil) at baseline (after ACL-injury prior to surgery) and 2-years after ACL reconstruction, and cartilage T1ρ and T2 relaxation times (resolution: 0.56*0.56*4mm; T1ρ spin-lock frequency 500Hz, Time of spin-lock: 0/10/40/80ms; T2 preparation TE: 2.9/13.6/24.3/45.6ms) were quantified in six compartments (medial/lateral femoral condyle, medial/lateral tibia, trochlea and patella) using methods developed previously.[3] Pearson correlation coefficients were calculated between NMR and immunoassay measures. Principal Component (PC) analysis was performed on NMR measures before a linear regression model was applied to evaluate if NMR measures predict cartilage T1ρ and T2 progression from baseline to 2-year follow-up. Age, gender, BMI and days from injury to surgery were adjusted in the model. Significance was defined with P < 0.05.Results
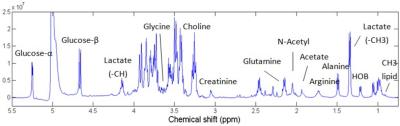
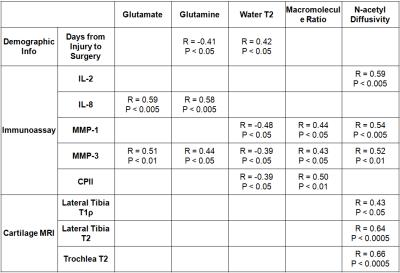
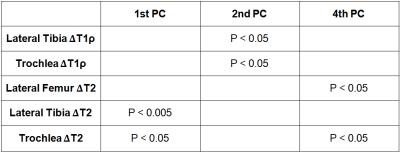
A representative 1H HR-MAS NMR spectrum of ACL-injured synovial fluid is illustrated in Fig.1. The concentrations of glutamate and glutamine by NMR were significantly correlated with the inflammatory cytokine IL-8 and the cartilage degradation enzyme MMP-3 by immunoassay (Table 1). Macromolecule ratio (signal intensity of macromolecules divided by total intensity of the whole spectrum)[2] and water T2 by NMR were found correlated with MMP-1, MMP-3, and the cartilage synthesis product CPII by immunoassay. The diffusion coefficient of N-acetyl by NMR was correlated with IL-2, MMP-1, and MMP-3 by immunoassay. Significant correlations were found between N-acetyl diffusivity and cartilage T1ρ of lateral tibia, and cartilage T2 of lateral tibia and trochlea (Table 1). Linear regression to T1ρ and T2 progression from baseline to 2-year indicated that the first PC (dominated by water T2 and glucose concentration) and the 4th PC (dominated by water D and N-acetyl concentration) predicted cartilage T2 progression, while the 2nd PC (dominated by water T1 and acetate concentration) predicted cartilage T1ρ progression (Table 2).Discussion and Conclusion
Novel NMR markers, including macromolecule ratio, the concentrations of glutamate and glutamine, N-acetyl diffusivity, and synovial fluid water T2, have been correlated with inflammatory cytokines and enzymes in synovial fluids after ACL injury, and more interestingly, also correlated with cartilage T1ρ and T2 cross-sectionally (at baseline) and longitudinally (progression from baseline to 2-year). The correlation between NMR measures and cartilage T1ρ and T2 was primarily found in the lateral side and trochlea cartilage, suggesting the lateral side cartilage will have an accelerated degradation if there is a combination of initial injury and high pro-inflammatory response. The results from this study provided quantitative evidence of initial inflammation and longitudinal cartilage degradation after acute injury, which will help to design novel treatment strategies to slow and prevent PTOA development. NMR profiling can be used to help select cohorts for novel interventions, and be used as a sensitive and specific outcome measure for treatment response.Acknowledgements
The study was supported by NIH P50 AR060752 Pilot grant, P30 AR066262 feasibility grant and Arthroscopy Association of North America Research Grant. The authors would like to thank Stephanie Taylor and Favian Su for their help in synovial fluid sample collection and MRI data processing, and Janet L Huebner and Thomas V Stabler for help with immunoassay analyses.References
[1] Shet K et al. NMR Biomed 2012; 25 (4): 538-544. [2] Xu K et al. ISMRM 2016. [3] Su F et al. Osteoarthr Cartilage 2013; 21 (8): 1058-1067. [4] Roberts HC et al. J Bone Joint Surg Br 2012; 94-B: SUPP XVIII 8. [5] Lehtinen P et al. Connect Tissue Res. 1978; 6(3): 155-159. [6] Anandacoomarasamy A et al. J Rheumatol 2008; 35 (4): 685-690.
Figures