1140
Magnetic Resonance Elastography of Emphysematous Rat Lung in vivo1Imagerie par Résonance Magnétique Médicale et Multi-Modalités (UMR8081) IR4M, CNRS, Univ. Paris-Sud, Université Paris-Saclay, Orsay, France, 2Centre de Recherches Biologiques, CERB, Baugy, France
Synopsis
Emphysema is a chronic respiratory disease, which has become the fifth most common cause of death worldwide. Emphysema is characterized by alveolar wall destruction, leading to distal airspace enlargement and decreased elastic recoil. The altered structure and function of the lung is related to modified mechanical properties of pulmonary tissues, which are difficult to probe in vivo by standard techniques. Magnetic Resonance Elastography (MRE) allows to characterize the mechanical properties correlated with lung physiopathologies. Here, we implemented MRE lung imaging in vivo on emphysematous rat models.
INTRODUCTION
Emphysema is a chronic respiratory disease, which has become the fifth most common cause of death worldwide. Emphysema is characterized by alveolar wall destruction, leading to distal airspace enlargement and decreased elastic recoil1. The altered structure and function of the lung is related to modified mechanical properties of pulmonary tissues, which are difficult to probe in vivo by standard techniques. Magnetic Resonance Elastography (MRE) allows to characterize the mechanical properties correlated with lung physiopathologies. Here, we implemented MRE lung imaging in vivo on emphysematous rat models.METHODS
Animal model: Seven female Wistar rats, (192±14) g, were endotracheally-instilled with Porcine Pancreatic Elastase (PPE; 25UI/100g BW) and two control rats, (195±4) g, with saline (0.2 mL/rat).
Administration protocol was carried out with a MRI-compatible Small Animal Gas Administration System (SAGAS)2, controlling breathing cycles and ventilation volumes. Mechanical ventilation was tuned to match individual free-breathing patterns (45-60 breath/min).
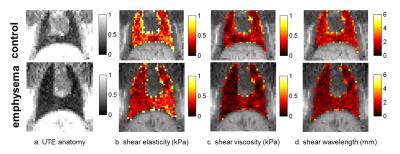
Imaging protocol was implemented on a clinical 1.5 T [Philips Achieva] with a 47 mm-diameter receiver coil. Rats, in supine position, were tracheotomised and anaesthetized (isoflurane-air or O2) through SAGAS with an intratracheal catheter. Two 3D-GRE radial acquisitions (TR/TE1/TE2 =15/0.4/1.4 ms) were used for R2* mapping and 3D isotropic T1W-UTE radial acquisition3 (TR/TE=14/0.4 ms, α=30º, 0.5 mm3 voxel, Tacq=7.5 min) was performed to characterize lung tissue. Pressure waves were generated by a remote loudspeaker at 236 Hz and transmitted to the lung along a waveguide4, with a silicone hose placed against the thoracic cage. MRE measurements using a multi-slice spin-echo to probe the whole lung with 1mm3-isotropic voxel, FOV=(64×40×35) mm, TR/TE=946/21 ms and 879 Hz bandwidth-per-pixel. Four snap-shots of the displacement field, encoded by a 21mT/m bipolar motion-sensitizing gradient, were acquired during the oscillatory cycle along the three spatial directions. Shear wavelength (λ ), elasticity (Gd) and viscosity (Gl) moduli distribution maps were reconstructed by 3D inversion method5.
Image analysis: Coil-sensitivity was corrected on UTE image by a conjugate-gradient method. Lung parenchyma density was assessed from sensitivity-corrected UTE, by considering surrounding tissue density equal to 1g/mL. Lung segmentation was performed by histogram-based method using GRE and UTE measurements3 and provided lung volume estimation. UTE image was further interpolated to provide density maps for MRE reconstruction. To assess regional distribution, lung was divided into 8 equal volumes along 3 anatomical directions, left/right, anterior/posterior and head/feet. Shear wavelength and viscoelasticity in each region of the lung were calculated. Corresponding elastography parameters in liver were compared for reference. Group analysis was expressed as mean ± standard error of the mean (SEM). Unpaired two-tail t-tests with a significance level of 0.05 were performed.
RESULTS
Lung parenchyma density is (0.45±0.08)g/mL in control and (0.30±0.04)g/mL in emphysematous rats. Lung volume is (5.63±1.52)mL for control and (7.21±1.22)mL for emphysematous lungs. Lung parenchyma R2* is (598.31±106.42)s-1 in control and (708.80±34.58)s-1 in emphysematous models.
In the liver, control and emphysematous rats show comparable dynamic shear modulus Gd (2.48±0.27)kPa vs. (2.61±0.08)kPa and loss shear modulus Gl (1.78±0.2)kPa vs. (1.65±0.19)kPa. In the lungs, emphysematous rats show ~30% lower mean viscoelastic moduli with Gd (0.47±0.03) kPa vs. (0.65±0.1) kPa and Gl (0.26±0.02) kPa vs. (0.39±0.09) kPa, as well as lower λ (1.71±0.1)mm vs. (2.5±0.36)mm (Fig.1). Regionally, Gl show a significant difference in emphysematous rat lungs on the right-anterior-feet region (0.32±0.01) kPa vs. (0.25±0.02) kPa (p<0.05) and λ on the left-anterior-feet region (2±0.03)mm vs. (1.57±0.09)mm (p<0.01).
DISCUSSION AND CONCLUSION
Lung SE-MRE was applied to mechanically-ventilated emphysematous rats. With a properly weighted density map, shear moduli maps were shown to be sensitive to pulmonary regional function. Inferred mean shear dynamic moduli in control lungs agree with values formerly-obtained by hyperpolarized helium-3 MRE7. The observed significant differences of loss shear modulus and wavelength on the anterior/feet region may be related to the regions which are likely more affected by emphysema8. 3D UTE and GRE were used to characterize lung density, volume and R2*. Measured lung density was close to CT reference7, with a lower density6 and enlarged volume1 in emphysematous lungs. An increase of R2* was observed in emphysematous model, which can be related to the enlarged airspaces therefore increased air-tissue interface. The apparent transverse relaxation R2* within the lungs could be further correlated to viscoelastic properties to localize the diseased tissues and histopathological information could provide supplementary confirmation. Motion correction may be further needed to improve MRE precision, combined with shortened TE to increase SNR. Here hydrogen MRE is an interesting non-invasive tool for diagnosis and treatment follow up of chronic respiratory diseases.Acknowledgements
This work is part of the OxHelease project (grant ANR-11-TecSan-006-02) and is supported by the Physics and Imaging for Medicine program at Université Paris-Saclay. Imaging was performed on the 1.5 T MRI platform at SHFJ (Orsay, France), partly funded by France Life Imaging (grant ANR-11-INBS-0006).References
1. Snider GL et al., Am Rev Respir Dis. 1985. 2. Wang H et al., ISMRM 2016; P1610. 3. Wang H et al., MRM 2015. 4. Maître X et al., ISMRM 2011; P3489. 5. Sinkus R et al. MRM 2007. 6. Olsson LE et al., J Magn Reson Imaging 2007. 7. Santarelli R et al., ISMRM 2013; P4117. 8. Matsuoka S et al., Radiographics 2010.Figures