1131
Evaluating the Influence of B1-Shimming on Contact-free Cardiac Gating using Scatter of a Parallel Transmit Coil at 7T MRI1Oxford Centre for Magnetic Resonance, University of Oxford, Oxford, United Kingdom
Synopsis
We propose a contact-free cardiac MRI triggering method based on reflected power measurements that requires no additional hardware other than that provided with a commercial parallel transmit (pTx) MRI scanner and evaluate the influence of B1+ shim on the cardiac information extracted. Time series of scattering matrices of the pTx monitoring system were used with random, uniformly distributed phases to simulate B1+ shims. Preliminary results in 7T MRI are shown with successfully, retrospectively gated 2D-CINE images using the proposed method.
Purpose
ECG remains the go-to method for cardiac MRI synchronisation but requires preparation, electrodes and sometimes expert adjustment. An alternative is proposed requiring no additional hardware other than that provided with a commercial parallel transmit (pTx) MRI scanner. We compare monitoring the scattering matrix and monitoring only scatter from an excitation RF-pulse with a range of B1+ shimming modes.Methods
We used a 7T MRI Scanner (VB17, Step 2.3, Siemens, Erlangen, Germany), equipped with 8-channel pTx. The local SAR monitor consists of 8 directional couplers (DICOs) connected on the transmission lines of a TEM cardiac transmit/receive coil1. The relationship between all channels of an N-port pTx coil is described by the scattering matrix(S). If the objects and their complex permittivity change within these RF-fields, this is reflected as a change in scattering2. We model S(t) as two components, one constant (Sexp) and one resulting from cardiac motion ($$$\triangle$$$Scardiac). For the case where S is resolved, we can measure $$$\triangle$$$Scardiac. However, during an excitation RF-pulse the returned voltage $$$\vec{V}_{ret}(t)$$$, which is measured by the DICOs, is a function of S(t) and the amplifiers forward voltage $$$\vec{V}_{fwd}(t) $$$ and the applied B1+shim ($$$\vec{A}$$$) as follows:
$$\vec{V}_{ret}(t)=\left( S_{exp}+\triangle S_{cardiac}(t)\right)\left[\vec{A}⨀\vec{V}_{fwd}(t)\right]$$
If Sexp is measured before a scan, we can remove expected interplay between channels and normalise by an element wise vector division (⊘) to form a vector ($$$\vec{Γ}(t) $$$) that is sensitive to cardiac motion as follows:
$$\vec{Γ}(t)=\vec{V}_{ret}(t)⊘S_{exp}\left[\vec{A}⨀\vec{V}_{fwd}(t)\right]=\triangle S_{cardiac}(t) \left[\vec{A}⨀\vec{V}_{fwd}(t)\right]⊘S_{exp}\left[\vec{A}⨀\vec{V}_{fwd}(t)\right]+\widehat{u}$$
$$$\vec{Γ}(t)$$$ is utilised as a vector of 8 real and 8 imaginary components and $$$\widehat{u}$$$ is a unit vector.
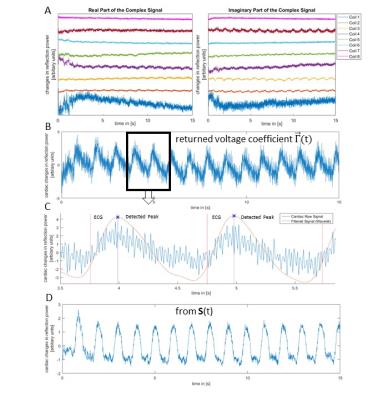
To extract a cardiac measure from either $$$\vec{Γ}(t)$$$ or S(t), the following method was applied: measurements were temporally de-trended and the cardiac signal was extracted using an independent component analysis of cardiac band pass filtered signals. The cardiac component was identified by a Welch power spectrum density estimate with highest power in the cardiac frequency band (f=0.6-2.4Hz) and the cardiac mixing vector was determined. The cardiac signal was analysed using a multiresolution discrete wavelet transform (DWT) with a maximal overlap. A mother wavelet with (N=5) vanishing moments of symlets was used and the mean heart rate was approximated using a fast-Fourier transform. The resulting dominate heart frequency DWT was chosen and a cardiac trigger event was determined using a peak detection of this signal (an example is shown in Figure 1 for $$$\vec{Γ}(t)$$$ and S(t).
Data acquisition
An S(t) monitoring pulse sequence was created to transmit a frequency multiplexed (2kHz channel spacing) 5ms Gauss RF-pulse every TR=10ms in 6 healthy adults (Male, age= 24-39) during a 15s breath-hold in compliance with our ethical regulations. ECG was recorded during all measurements.
Simulation of B1-Shimming on RF-excitation used for MRI
The time series of S-Matrices was averaged to determine Sexp. 10,000 vectors with random, uniformly distributed phases were generated to emulate potential B1+ shim sets ($$$\vec{A_{i}}$$$). The S-matrix time-series were used to determine $$$\vec{Γ}(t)$$$ for each $$$\vec{A_{i}}$$$ to simulate reflected power measurements based only on excitation RF-pulses used in B1+ shimming. The time of the cardiac trigger event (peak) was determined for each B1+ shim and each S-matrix combination. Further, one 2D-GRE cardiac cine (product sequence) was acquired to test retrospective gating with the proposed method using $$$\vec{Γ}(t)$$$ based on the eight return voltages measured during transmit.
Results
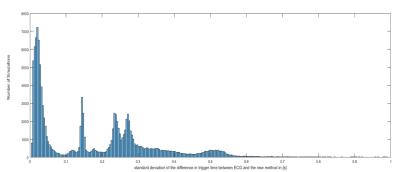
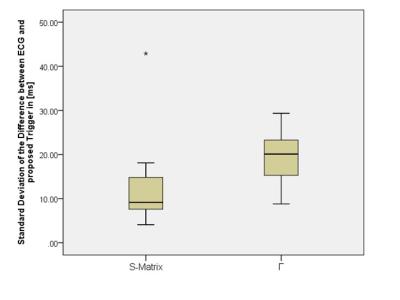
The standard deviation of the difference in trigger time between ECG and the new method (jitter) using the monitoring pulses and S(t) was 12.58±9.1ms. For simulated B1+ shimming and the use of $$$\vec{Γ}(t)$$$ , it varied considerably as a function of shim, see Figure 2, with the dominant modes having a jitter of 19.43±6.75ms and a number of RF shim settings providing no cardiac trace.
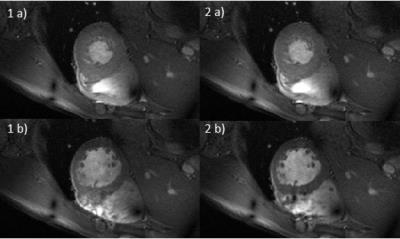
Two time frames from the retro-gated cine, are shown in figure 4 and compared with ECG gating.
Discussion
We have shown that it is possible to extract cardiac gating information from the reflected voltage of an imaging RF-pulse in a pTx setup. However, it is dependent on the B1+ shim, with some B1+ shims yielding no viable cardiac trace. Therefore, during selection of $$$\vec{A_{i}}$$$ (B1+ shim), knowledge of $$$\triangle S_{cardiac}(t)$$$ should be taken into account.
The suitability of this method for cardiac gating at 7T has been shown with preliminary results on retrospectively gated 2D-CINE images without any pulse sequence modification. The images were acquired without any further external hardware nor sequence changes.
Conclusion
B1+ shim influences the cardiac trace extracted by a new method for contact-free cardiac synchronisation in MRI but can still be used for successful gating on pTx cardiac MRI scanners without additional hardware.Acknowledgements
This work was supported by funding from the Engineering and Physical Sciences Research Council (EPSRC) and Medical Research Council (MRC) [grant number EP/L016052/1], the Clarendon Fund and Keble College de Breyne Scholarship.References
1. Snyder, C. J. et al. Comparison between eight- and sixteen-channel TEM transceive arrays for body imaging at 7 T. Magn. Reson. Med. 67, 954–964 (2012).
2. Buikman, D., Helzel, T. & Roeschmann, P. The rf coil as a sensitive motion detector for magnetic resonance imaging. Magn. Reson. Imaging 6, 281–289 (1988).
Figures