1127
Panning for Salt: One Millimeter Resolution In Vivo Sodium MRI of the Human Eye at 7.0 Tesla Using a Six Channel Transceiver Array1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin, Germany, 2MRI.TOOLS GmbH, Berlin, Germany, 3Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 4Division of Medical Physics in Radiology, German Cancer Research Centre (DKFZ), Heidelberg, Germany, 5Department of Ophthalmology, University of Rostock, Rostock, Germany, 6Institute for Diagnostic Radiology and Neuroradiology, University Medicine Greifswald, Greifswald, Germany, 7Institute for Physiology, Charité University Medicine, Berlin, Germany, 8Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association
Synopsis
Sodium quantification in the eye may be a valuable aid not only in diagnosis of ocular diseases, but in follow-up after proton therapy of eye tumors. Recognizing this potential, this is the first report on high fidelity in vivo sodium (23Na) MRI of the human eye at 7.0 Tesla. To achieve this goal a six-channel 23Na transceiver array was designed, simulated, built and validated in phantoms. The in vivo studies demonstrated the feasibility of 1 mm isotropic spatial resolution 23Na MRI of the eye and provided encouragement for clinical studies.
Purpose
Cataracts and ocular glaucoma, two main causes of blindness, affect almost 30 million people in the US and the numbers are rising 1. New forms of diagnosis are needed to assess individual risk and design personalized treatments. Sodium ions play a key role in processes in the eye including the active maintenance of the Na+/K+ gradient between lens and vitrous humour (VH). There have been several reports of increases in Na+ concentration in cataractous lenses 2,3. Ex vivo studies reported diminished Na+ concentration in the aqueous humour (AH), which probably contributes to the pathophysiology of bilateral acute angle closure glaucoma 4. Visualizing tissue sodium (23Na) concentration (TSC) could provide helpful information about this critical parameter in pathophysiological processes, but this is beyond the reach of current clinical MRI. Ultrahigh-field MRI offers a unique potential to visualize 23Na non-invasively for diagnostic purposes. The aim of this project is to achieve high spatial-resolution 23Na imaging of the human eye in vivo at 7.0 T and to determine its value for understanding, diagnosis and treating diseases that affect this intricate tissue.Methods
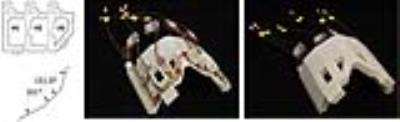
We designed, simulated, built, validated and applied a six-channel transceiver array customized for ophthalmic 23Na MRI at 7.0 T (f0=78.6 MHz). The analysis of RF circuits was performed with Advanced Design System (Keysight EEsof EDA, CA, US). Adjacent elements of the array shared a common conductor and were decoupled with trimmer capacitors (Voltronics, MD, US). Non-adjacent elements, as well as the two elements to be placed right above the eyes, were decoupled inductively. Electromagnetic field (EMF) and specific absorption rate (SAR) simulations were performed in CST Studio Suite (CST AG, Darmstadt, Germany). The transmit field (B1+) generated by the array was optimized using a customized MATLAB routine (MathWorks, MA, USA) to reduce SAR and to balance B1+ uniformity and efficiency in the region of interest (ROI), covering both eyes of the human voxel model Duke 5. For validation of the B1+ shimming results derived from the simulations, phantom imaging studies were performed using a whole-body 7.0 T MR system (Magnetom, Siemens Healthcare, Erlangen, Germany). In vivo sodium imaging was performed using a density adapted 3D projection reconstruction technique 6.Results
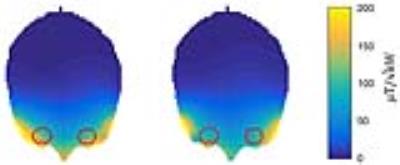
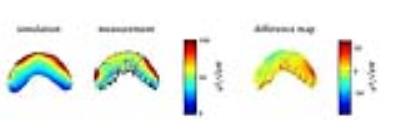
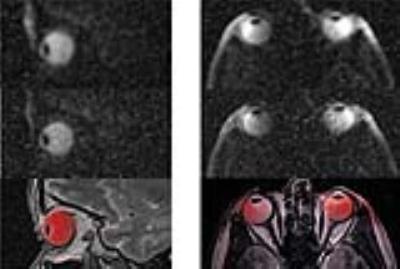
The RF coil is light weight and conforms closely to an average anterior human head (Figure 1). Reflection coefficients of the individual elements were ≤ -21 dB. Transmission coefficients were ≤ -13 dB.Transmission field uniformity was examined for: equal phase for all channels (EP) and optimized phase setting (PO) (Figure 2). Assessment of B1+ homogeneity derived from the EMF simulations for a ROI covering the eyes yielded a standard deviation of SD=18 % for EP and SD=12 % for PO. Maximum SAR10g - 4.84 W/kg was found for EP (Figure 3). The PO afforded a SAR reduction around the nose and provided SAR10gmax - 1.87 W/kg which is very well within the RF power deposition limits governed by the IEC guideline 7. B1+ mapping using a double angle method and a cylindrical phantom provided fair agreement with simulation data for EP (Figure 4). The in vivo feasibility study yielded high spatial resolution (nominal isotropic resolution=1.45 mm3 and 1.0 mm3, scan time=10 min and 14 min) 23Na MR images of the human eye (Figure 5) at 7.0 T. The VH provided mean SNR ≈18 (1.45mm3) and ≈9 (1.0mm3). The mean SNR of the lens was ≈9 (1.45mm3) and ≈6 (1.0mm3). Along a line connecting the center of the lens with the retina a signal intensity change of 12% was observed within VH.Discussion and Conclusion
This is the first report of a dedicated RF transceiver array to image sodium in the human eye in vivo. Now we will explore the efficacy of the method in healthy subjects and in a feasibility study involving patients. Na+ concentrations in the eye likely rise before the appearance of an opaque lens, and studies of this process could add a new dimension to our understanding of cataracts. Glaucoma is linked to persistent, elevated intraocular pressure (IOP), which is controlled by the outflow of fluid from VH to AH. This is thought to alter the Na+ concentration between the AH and VH. Diminished sodium AH levels, contributing to bilateral acute angle closure glaucoma have been reported in ex vivo studies of glaucoma, as well as reports of increases in TSC in brain tumors, which suggests that the same effect might occur in ocular melanoma 8. These findings indicate that sodium quantification in the eye may be a valuable aid not only in diagnosis, but in follow-up after post-proton therapy for eye tumors 9.Acknowledgements
No acknowledgement found.References
1. Eye Disease Statistics, National Eye Institute, Mar 2014.
2. Garner WH, et al., Proc Natl Acad Sci USA. 1986 Mar;83(6):1901-5.
3. Chandorkar AG, et al., Indian J Ophthalmol. 1983 May;31(3):262-4.
4. Chen SH, etl al., BMJ Case Rep. 2014 Dec 4;2014.
5. Graessl A, et al., Invest Radiol. 2014 May;49(5):260-70.
6. Nagel AM, et al., Magn Reson Med. 2009 Dec;62(6):1565-73.
7. IEC 60601-2-33 ed. 3.
8. Ouwerkerk, et al., Radiology. 2003 May;227(2):529-37.
9. Haneder S, et al., Siemens Magnetom World, 2013 Apr.
Figures