1125
Abdominal Imaging with Heterogeneous Radiofrequency Fields at 7 Tesla1Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University School of Medicine, New York, NY, United States, 3The Sackler Institute of Graduate Biomedical Sciences, New York University School of Medicine, New York, NY, United States, 4Siemens Medical Solutions USA, Inc., Siemens Healthineers, New York, NY, United States
Synopsis
Body imaging using ultra-high field systems operating at 7T or more is extremely challenging due to the non-uniformities in the excitation field. Traditionally, these field variations are seen as a nuance that must be calibrated out, leading to a formidable engineering challenge. Recently, a new paradigm was proposed which deliberately interweaves multiple uncalibrated non-uniform RF fields into the scan. In this work we demonstrate these principles in-vivo and show that it is possible to obtain artifact free cross-sectional quantitative maps of the abdomen at 7T across a variety of different subject sizes without the need for any specific calibrations.
Purpose
Body imaging using Ultra-high field systems is extremely challenging due to the non-uniformities in the excitation (B1+) field. Even routine abdominal scan at 3 Tesla are frequently hampered by B1+ artifacts [1]. Traditionally, these B1+ field inhomogeneities are seen as an engineering challenge, leading to specialized coil designs [2,3, among many others] and advanced calibration mechanisms [4,5]. Recently, a new paradigm was proposed which deliberately interweaves multiple uncalibrated non-uniform RF fields into the scan [6]. The numerical simulations presented in that same paper also suggest that this new approach could enable rapid quantitative abdominal imaging even at ultra-high field strengths. In this work we demonstrate these principles in-vivo and show that it is indeed possible to obtain artifact free cross-sectional quantitative maps of the abdomen at 7 Tesla across a variety of different subject sizes without the need for any specific calibrations.Methods
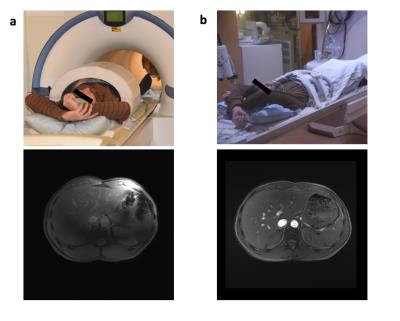
Three subjects of different sizes were scanned at 7 Tesla, small, medium and large (body mass index of 18, 23, and 28 respectively). All experiments were performed using our MAGNETOM 7T equipped with 8 parallel transmit channels (Siemens Healthineers, Erlangen, Germany). An in house developed stand-off body coil array was used (Fig. 1a), consisting of an 8-element transceive dipole array complemented with an 8-element birdcage array for reception. The study was approved by our institutional review board.
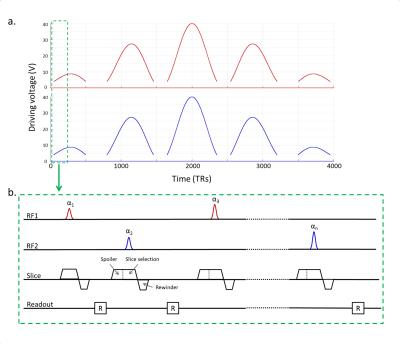
The power limit for in-vivo imaging with the prototype 7T body coil was based on the worst case assessment [7]. A special low-power Plug and Play MR-Fingerprinting (PnP-MRF) [6] sequence was implemented to enable breath-hold imaging. Because accurate estimation of the transverse relaxation time (T2) requires a substantial refocusing component, we employed an RF spoiled design that is sensitive only to the proton density (PD), the longitudinal relaxation time (T1), and B1+. The sequence consists of 4000 excitations with driving voltages ranging between 0V and 40V/channel (Fig.2a). The coil configuration alternates every TR between the circularly polarized (CP) mode and gradient mode. A golden-angle radial readout [8] is used and the spoiler gradient is applied in the slice direction (Fig.2b). The reconstruction was performed as described in [6].
Results & Discussion
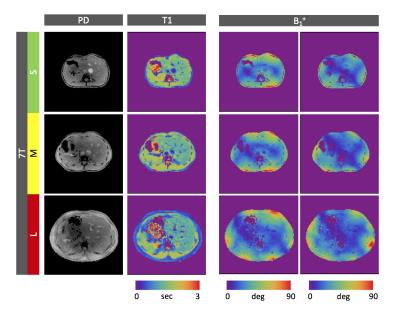
The B1+ field produced by the standard body coil still suffices to image our medium size subject at 3 Telsa (Fig.1b). At 7 Tesla, however, the CP-mode results in strong B1+ variations and RF voids that completely change the signal and contrast distribution (Fig.1a). Figure 3, on the contrary, shows the PnP-MRF results obtained in each of the three different subjects. Although the body sizes are very different, the coil modes remain complementary, enabling the robust quantification of PD and T1. The self-calibrating nature of the sequence automatically separates out and quantifies the B1+ distributions of both coil modes (Fig.3 last two columns), thus providing a simple and efficient workflow without the need for advanced patient specific calibrations. In fact, the allowed dynamic B1+ range is so large that the sequence could simply be played out with a fixed transmit reference voltage without any transmit voltage calibration.
In this proof of principle experiment, the multi-parametric maps were limited to PD, T1 and B1+. From a sequence design point of view, it will be relatively straight forward to incorporate T2 quantification. However, this will likely need excitations with a more substantial refocusing component and thus larger flip-angles. We hope to facilitate this by moving towards a less conservative safety assessment using a virtual observation points based specific absorption rate monitoring system. Alternatively, the coil could also be driven using a butler matrix in combination with a switch. A higher power-limit could also be applied to speed up the sequence by reducing the RF pulse duration and TR.
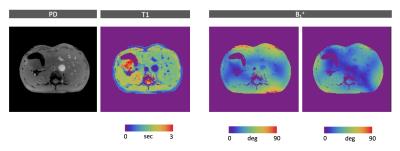
In general, there is also a lot of room for optimization on the sequence side. Jiang et al [9], for example, showed that the number of RF pulses can be reduced significantly using the quick echo splitting technique. On the reconstruction side, advanced iterative techniques can help to reduce the scan-time or increase the resolution [10]. Nevertheless, even without such advanced techniques we found that the in-plane resolution could easily be doubled (Fig.4).
Conclusion
We have demonstrated experimentally that PnP-MRF can indeed enable a plug and play workflow for abdominal imaging at ultra-high field strengths. Until now, research systems operating at 7 Tesla have predominantly focused on brain and extremity imaging because of the severe RF inhomogeneities encountered in the body. We showed that PnP-MRF has the potential to alleviate these challenges, and thereby open up a new route towards robust, quantitative, whole-body MRI for ultra-high-field systems.Acknowledgements
This work was supported in part by NIH R21 EB020096 and NIH R01 AR070297 and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
1. Bernstein, M. A., Huston, J. & Ward, H. A. Imaging artifacts at 3.0T. J. Magn. Reson. Imaging 24, 735–746 (2006).
2. Vaughan, J.T., Snyder, J.C.,DelaBarre, L.J., Bolan, P.J., Tian, J., Bolinger, L., Adriany, L., Andersen, P., Strupp, J., & Ugurbil K. 7 T Whole Body Imaging: Preliminary Results. Magn. Reson. Med. Jan; 61(1): 244–248 (2009).
3. Brunner, D.B., De Zanche, N., Fröhlich, J., Paska, J. & Pruessmann, K.P. Travelling-wave nuclear magnetic resonance. Nature 457, 994-998 (2009)
4. Katscher, U., Bo ¨rnert, P. & Leussler, C. Transmit sense. Magn. Reson. Med. 49, 144–150 (2003).
5. Zhu, Y. Parallel excitation with an array of transmit coils. Magn. Reson. Med. 51, 775–784 (2004).
6. Cloos, M. A., Knoll, F., Zhao, T., Block, K. T., Bruno, M., Wiggins, G. C., & Sodickson, D. K. Multiparametric imaging with heterogeneous radiofrequency fields. Nat. Commun., 7 (2016).
7. Cloos, M.A., Anon. L., Chen, G., Wiggins, G.C., & Sodickson, D.K. Rapid RF Safety Evaluation for Transmit-Array Coils. In the Proceedings of the 21th Annual Meeting of the ISMRM, Salt Lake City, USA, 2013, p0286.
8. Winkelmann, S., Schaeffter,T., Koehler,T., Eggers, H. & Doessel, O. An Optimal Radial Profile Order Based on the Golden Ratio for Time-Resolved MRI. IEEE Transactions on Medical Imaging, 26(1):68–76 (2007).
9. Jiang, Y., Ma, D., Jerecic, R., Duerk, J., Seiberlich, N., Gulani, V. & Griswold M.A. MR fingerprinting using the quick echo splitting NMR imaging technique, Magn. Reson. Med. ( early view).
10. Assländer,J., Cloos, M.A., Knoll, F., Sodickson, D.K., Hennig, J. & Lattanzi, R. Low Rank Alternating Direction Method of Multipliers Reconstruction for MR Fingerprinting. preprint, page arXiv:1608.06974.
Figures