1124
Initial experiences with a fully-removable whole-body birdcage transmit coil and 16-element receive array for cardiac 31P-MRS at 7TLadislav Valkovic1, Iulius Dragonu2, Karsten Wicklow2, Ulrich Joerg Fontius2, Salam Almujayyaz3, Alex Batzakis3, Liam Young1, Lucian AB Purvis1, William T Clarke1, Tobias Wichmann4, Titus Lanz4, Stefan Neubauer1, Matthew D Robson1, Dennis WJ Klomp3,5, and Christopher T Rodgers1
1Oxford Centre for Clinical MR Research (OCMR), RDM Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom, 2Siemens Healthcare GmbH, Erlangen, Germany, 3MR Coils BV, Zaltbommel, Netherlands, 4Rapid Biomedical GmbH, Rimpar, Germany, 5Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
This abstract describes our experiences implementing a volume transmit, local receive setup for cardiac 31P-MRS on a Siemens research 7T MRI scanner. Two strands of development work have been performed in tandem: (i) development of a fully removable whole-body transmit RF-coil and testing with the standard 8kW RFPA and SAR monitoring and combined with a 16ch receive array, and (ii) integration of a 35kW RF power amplifier, a new energy chain, and adapted SAR monitoring.
Introduction:
31P-MRS plays an important role in the assessment of tissue energy metabolism in vivo, through measurement of phosphorus-containing metabolites, e.g., phosphocreatine (PCr) and adenosine-triphosphate (ATP). In the human heart, PCr/ATP ratio has been identified as a valuable pathology marker.1,2 However, 31P-MRS suffers from low intrinsic SNR which can be partially mitigated by using ultra-high field, e.g. 7T. But strong B1+ inhomogeneity of surface transmit coils at 7T makes acquisition of spatially-resolved 31P-MRSI spectra across the heart challenging. Recently, a whole-body-sized RF birdcage coil was integrated into a Philips 7T MR system and shown to generate homogeneous RF excitation for 31P-MRSI examinations across the human body.3 However, that coil was operated only in transceiver mode, powered by a 4kW amplifier, and required removal of the magnet covers and patient bed for installation/removal.This study reports initial results of a collaborative project to design, build and test a new, fully-removable whole-body 31P coil for use on a Siemens 7T MR scanner (Siemens Healthcare, Erlangen, Germany), and to use it in conjunction with a 16-element receive array.4 We also present preliminary phantom results from integrating a 35kW RF power amplifier, typically used for 1H-MR at 3T.
Methods:
The fully removable design of the whole-body coil provided by MR Coils (MR Coils, Zaltbommel, Netherlands) is depicted in Figure 1. The lower part of the coils is integrated into an extension of the patient table, which rests on custom-built support rails and connects to the normal patient table at the service-end of the magnet. The upper part is detachable for ease of access. The local body SAR efficiency and mean B1+ in the chest were simulated using CST Studio Suite 2016 software and Gustav model (CST). In vivo measurements were performed using the standard Siemens 8kW RF power amplifier, while we await completion of the high-power energy chain and approval by local safety committee.The coil performance was tested in a two compartment phantom consisting of a NaCl(aq) filled container and a 2cm cube, filled with KH2PO4(aq), fixed at a 6cm depth. The SNR and peak B1 were compared between a quadrature coil5, 16ch receive array with a local transmit loop from Rapid Biomedical (Rapid Biomedical, Rimpar, Germany), whole-body coil in transceiver mode, and the combination of whole-body transmission and 16ch reception.
Three healthy volunteers were examined using two acquisition weighted 31P-UTE-CSI6 experiments with a 16x16x8 matrix and 500x500x400mm3 field-of-view. In total, 14 minutes 19 seconds were required to acquire 8 averages with TR=1s. Two non-adiabatic saturation bands (10ms duration) were used to suppress signal from chest and abdominal muscles in one of the measurements.
Finally, the peak output power at 120.3MHz of the MKS-S41 RF amplifier (MKS Inc, China), was tested using a 5ms pulse and a TR=101ms. To effectively use the SAR monitoring of the unmodified system, 1/11th of the peak RF output power was extracted and fed to the TALES RF power monitor (Siemens), and then into a 50Ω load. The coil’s “k-factor” (SAR/power) was scaled appropriately. A FLASH phantom image was acquired in 38s to demonstrate the feasibility of the setup.
Results/Discussion:
The simulated global body and local (10g) SAR efficiency of the designed whole-body coil were 0.29 and 3.6W.kg-1.μT-1, respectively, what is in good agreement with literature.3 The mean simulated B1+ per 1W excitation was 0.160±0.075μT for the heart and 0.122±0.068μT for liver. The SAR and B1+ maps are depicted in Figure 2, the B1+ maps show high transmit field homogeneity.Typical MR spectra acquired in human heart and liver are depicted in Figure 3, which also shows the effectivity of the saturation bands on the PCr signal intensity. The results of the SNR comparison are given in Table 1. The SNR of the 16ch receive array is lower in combination with the whole-body transmit coil than with the local transmit coil, mainly because the whole-body coil is at the moment not detuned in receive mode.
No significant droop during pulse was observed during the amplifier tests. The online SAR supervision using a fraction of the RF output power (Figure 4a) was successful, aborting scanning at the same actual power level as the unmodified system. A phantom image acquired with the whole-body coil in transceiver mode using the 35kW amplifier is depicted in Figure 4b.
Conclusion:
A fully-removable system for body 31P-MRSI was successfully designed and tested on a Siemens 7T scanner. Integrating a receive array significantly improves SNR. The feasibility of using a 35kW RF power amplifier and SAR monitoring on a fraction of the forward power has been demonstrated.Acknowledgements
This work was funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (grant #098436/Z/12/Z). The support by EPA Cephalosporin Fund, British Heart Foundation CRE and OCMR is also gratefully acknowledged.References
1. Neubauer S. Mechanisms of disease - The failing heart - An engine out of fuel. N Engl J Med 2007;356:1140-51.2. Bottomley PA. NMR Spectroscopy of the Human Heart. In: Harris RK, Wasylishen RE, eds. Encyclopedia of Magnetic Resonance. Chichester: John Wiley; 2009.
3. Loring J, van der Kemp WJM, Almujayyaz S, van Oorschot JWM, Luijten PR, Klomp DWJ. Whole-body radiofrequency coil for P-31 MRSI at 7T. NMR Biomed 2016;29:709-20.
4. Rodgers CT, Clarke W, Berthel D, Neubauer S, Robson M. A 16-element receive array for human cardiac 31P MR spectroscopy at 7T. In: ISMRM. Milan; 2014:2219.
5. Schaller B, Paritmongkol W, Magill A, Robson MD, Rodgers CT. Quadrature 31P and single 1H dual-tune coil for cardiac 31P-MRS at 7T. In: ISMRM2016.
6. Robson MD, Tyler DJ, Neubauer S. Ultrashort TE chemical shift imaging (UTE-CSI). Magn Reson Med 2005;53:267-74.
Figures
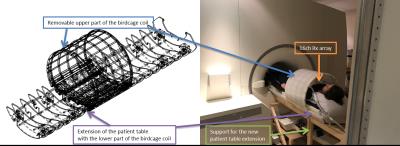
Figure 1: CAD rendering of whole-body 31P RF transmit
coil (left)
and the first iteration installed on our 7T MRI scanner (right).
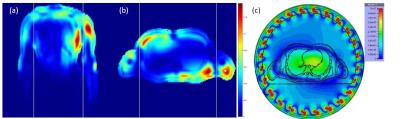
Figure 2: Electromagnetic field modelling results for the
whole-body coil. (a-b) 10g SAR showing hotspots in the chest wall and
shoulders, which are used to set a safe maximum RF dose allowed by the scanner.
Coronal view (a) and sagittal view (b). (c) Predicted B1+
efficiency. Note the uniformity of excitation across the heart and liver.
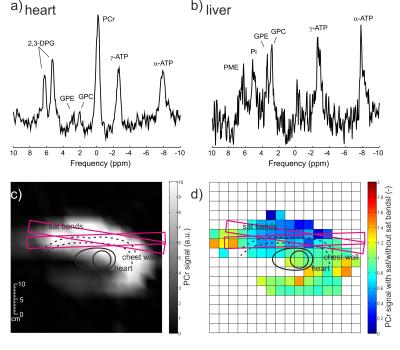
Figure 3: Initial
pilot data acquired from a volunteer using the whole-body transmit array
(operating with first-generation hardware limited to 6.5kW peak power) and a
16-element anterior cardiac receive array (Rapid Biomedical GmbH). Cardiac
spectrum (a), liver spectrum (b). (c-d) Images of phosphocreatine in oblique
view through cardiac mid-short-axis. The chest wall is clearly visible in (c).
In (d) is depicted a ratio of PCr signal from acquisition with and without the saturation
bands applied to reduce skeletal muscle signal.
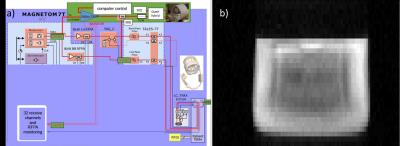
Figure 4: Schematic
of modifications to the Oxford 7T MRI scanner RF transmit and specific
absorption rate (SAR) monitoring system that assures we cannot burn a subject
(left). Image of 31P in a 10L bottle containing triphenylphosphite
obtained in 38s using the body coil for Tx and Rx (right).
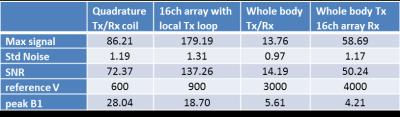
Table 1: Comparison
of the SNR, reference voltage and peak B1 between our coils/coil combinations