1121
Assessing Metabolic Intervention in Acute Myeloid Leukemia with a Glutaminase Inhibitor by Hyperpolarized Magnetic Resonance1Cancer Systems Imaging, MD Anderson Cancer Center, Houston, TX, United States, 2Bioengineering, Rice University, Houston, TX, 3Leukemia, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Acute myeloid leukemia (AML) is glutamine addicted cancer. We determined if hyperpolarized pyruvate could be utilized to detect in vivo metabolic changes in AML (OCI-AML3 cell line) bearing mice after CB839 (glutaminase inhibitor) treatment. We found a reduction of pyruvate to lactate conversion after treatment. In vitro analysis of OCI-AML3 reveal that NADH/NAD+ ratio, ATP, hydrogen peroxide levels and respiratory capacity reduce in CB839 treated cells compared to vehicle controls. Our data supports the hypothesis that in AML glutamine generates reducing equivalences by the citric acid cycle and inhibiting this process with CB839 reduces the rate of conversion of pyruvate to lactate.
Purpose
Acute myeloid leukemia (AML) is a hematopoietic malignancy which results in the accumulation and expansion of immature leukemia cells (blasts). In AML, glutamine dependence is increased.1,2 One of the critical steps in glutamine’s utilization is its conversion to glutamate through a glutaminase (GLS) enzyme.1,2 It was found using The Cancer Genome Atlas that the expression of GLS was within the upper 4% of all genes in the AML cohort.2 This increased utilization of GLS leads to the susceptibility of several AML cell lines to the small molecule CB839.1,2 CB839 is a nanomolar binder to both major isoforms of GLS1, has good oral bioavailability, and is in Phase I studies for solid tumors and leukemia.3 In this study, we utilized in vivo metabolic imaging to rapidly detect changes in metabolism and redox state after CB839 treatment in OCI-AML3 engrafted animals.Methods
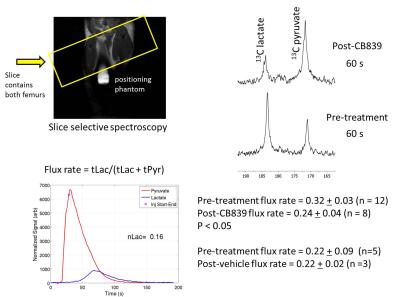
NSG mice were injected via the tail vein with human OCI-AML3 cells ~5 x 106 (luciferase and GFP expressing). After two to three weeks, animals were imaged using D-Luciferin and animals with high bioluminescence signal were utilized in hyperpolarized pyruvate experiments. Animals were injected with 200-250 μl of 80 mM of hyperpolarized 1-13C pyruvate. A series of single slice 13C spectra (placed over the leg, field-of-view 40 x 40 mm, slice-thickness 12-15 mm) were collected right after injection of hyperpolarized 1-13C pyruvate. A total of 90 transients (10 – 15o gauss pulse, 2048 points) were acquired with a 2 s time delay between each transient. Dynamic spectra were manually phased and line-broadening was applied (10 to 15 Hz). The area under the spectral peaks for pyruvate and lactate were integrated over the whole array. Animals were then allowed to recuperate from isoflurane anesthesia (30 minutes to several hours) and then orally gavaged with CB839 (200 mg/kg) or vehicle control. Animals were then re-imaged with hyperpolarized pyruvate 4 hours later. All imaging and spectroscopy were performed with either a dual tuned 1H/13C volume coil (OD: 75mm, ID:40mm) or in a combination 1H volume coil with a transmit/receive carbon-13 surface coil (OD: 20mm, ID:15 mm) placed directly on the femurs in a 7T Bruker Biospec horizontal bore MR scanner. Normalized lactate (nLac) ratio was calculated as lactate over the sum of pyruvate and lactate signals. In addition, biochemical assays and the Seahorse Bioanalyzer were utilized to determine the NADH/NAD+ ratio, hydrogen peroxide, metabolic profile and oxygen consumption of OCI-AML3 cell line grown in the presence and absence of 1 μM CB839.Results
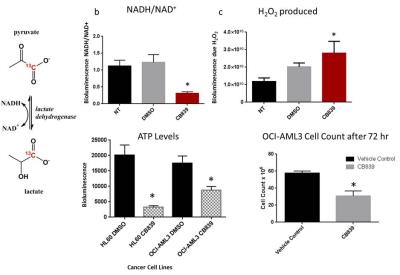
ATP levels and cell proliferation were significantly reduced in AML cell lines when incubated with 1 μM CB839 for 72 hours. CB839 treatment was found to reduce levels of glutamate (GLS metabolic product) by nuclear magnetic resonance and by liquid chromatography-mass spectroscopy (LC-MS)2 in OCI-AML3 cells. Glutaminase inhibition with CB839 caused a decrease of the basal and maximal respiratory capacity of the cells. Inhibition of glutaminase in vivo following exposure of mice with 200 mg/kg dose of CB839 showed a decrease hyperpolarized pyruvate to lactate conversion within the leg (0.32 + 0.03 (pre) to 0.24 + 0.04 (post) P < 0.05). To further understand the changes that occur in lactate levels with CB839 treatment, we measured the amount of lactate in the media and the intracellular metabolites of glutamate, lactate, and alanine in OCI-AML3 cells treated with 1 μM CB839. We found reduced lactate, glutamate and alanine levels in the treated cells. The levels of NAD+, NADH and hydrogen peroxide were measured using bioluminescence kits 12 and 72 hours after CB839 treatment respectively. A major reduction in the NADH/NAD+ ratio and an increase in hydrogen peroxide were observed in the CB839 treated OCI-AML3 cells. These results suggest that there is a change in the redox state of the cell after CB839 treatment and that the change in pyruvate conversion we observe after treatment is reflective of this.Discussion
In this study, we have observed a reduction in hyperpolarized pyruvate to lactate conversion just 4 hours after treating OCI-AML3 bearing animals with CB839. Our in vitro results reveal the reduction of NADH/NAD+ ratio in OCI-AML3 cells after treatment with CB839. Our data suggests this leads directly to the reduction of the conversion of pyruvate to lactate through the lactate dehydrogenase enzyme both in vitro and in vivo. Hyperpolarized pyruvate might be a method to measure directly the metabolic changes that occur with CB839 in AML patients or in patients with other malignancies, and can be used in the future to gauge the metabolic efficacy of the novel agents affecting metabolism in leukemia.Acknowledgements
We would like to thank Calithera Biosciences and specifically Dr. Susan Demo, Director of Translation Research at Calithera Biosciences, for all the discussions on the data. We would like to thank the CCSG-funded characterized cell line core facility at M.D. Anderson, small animal imaging facility and NMR facility (CA016672). We also thank Leukemia NCI SPORE DRP, Koch Foundation, NCI R25R21CA185536-02, CPRIT- RP140218, RP150701, MD Anderson IRG grants and MD Anderson startup for funding support.References
1 Jacque, N. et al. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood 126, 1346-1356, doi:10.1182/blood-2015-01-621870 (2015).
2 Matre, P. et al. Inhibiting glutaminase in acute myeloid leukemia: Metabolic dependency of selected AML subtypes. Oncotarget (Accepted ).
3 Gross, M. I. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther 13, 890-901, doi:10.1158/1535-7163.MCT-13-0870 (2014).
Figures