1118
Intravoxel incoherent motion diffusion-weighted MRI during chemoradiation therapy to monitor treatment response in human papillomavirus head and neck squamous cell carcinomaRamesh Paudyal1, Jung Hun Oh1, Nadeem Riaz2, Praveen Venigalla2, Jingao Li3, Vaios Hatzoglou4, Jonathan Leeman2, David Aramburu Nunez1, Yonggang Lu5, Joseph O. Deasy1, Nancy Lee2, and Amita Shukla-Dave1,4
1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiation Oncology, Memorial Sloan Kettering Cancer Center, NY, United States, 3Radiation Oncology, Jiangxi Cancer Hospital, Chile, 4Radiology, Memorial Sloan Kettering Cancer Center, NY, United States, 5Radiation Oncology, Washington University in St. Louis, MO, United States
Synopsis
This study aims to monitor treatment response in human papillomavirus (HPV) head and neck squamous cell carcinoma using pre- and intra-treatment (TX) week 1, 2 and 3 imaging metrics derived from intravoxel incoherent motion (IVIM) DW-MRI. An unsupervised hierarchical clustering with a distance based on the Pearson correlation coefficient was performed using the relative percentage changes in D, f and D* to investigate similarities among features and samples. D showed a significant increase during treatment in complete response (CR) group. A heat map generated from the unsupervised hierarchical clustering identified subtypes in HPV positive [+] HNSCC patients.
Purpose
Head and neck squamous cell carcinoma (HNSCC) was traditionally associated with behavioral risk factors, such as smoking and alcohol, but in the past decade, the human papillomavirus (HPV) infection has emerged as a novel etiologic agent of oropharyngeal carcinoma (1). HPV negative [-] tumors continue to have poor prognosis despite treatment intensification (2). In contrast, HPV-related tumors (HPV positive [+]) are associated with markedly improved outcomes and are now accepted as a distinct biological entity (3, 4). It is critical to identify patients who may be candidates for treatment de-intensification, including dose de-escalation in radiotherapy (3). Identifying patients with tumors exquisitely responsive to therapy would facilitate the next generation of clinical trials in HNSCC. DW-MRI has been utilized in tumor characterization and evaluation of treatment response in HNSCC (5, 6). The present study aims to monitor treatment response in HPV HNSCC using intra-treatment (TX) imaging metrics derived from IVIM DW-MRI. Additionally, to investigate similarities among features and samples, we used an unsupervised hierarchical clustering with a distance measure based on the Pearson correlation coefficient.Methods
IVIM DW-MRI data acquisition: Our institutional review board approved this prospective study and written informed consent was obtained from all eligible newly diagnosed HNSCC patients with neck nodal metastasis; diagnostic biopsies were tested for HPV status prior to the MRI study. Thirty-four HPV (30 HPV+ and 4 HPV-) HNSCC patients were included in the study. A total of 136 MRI studies were performed with anatomic and DW-MRI sequences (pre-TX, weeks 1, 2 and 3 intra-TX) and the patients were treated with chemo- and radiation therapy (dose 70Gy). MRI protocol consisted of multi-planar T1/T2 weighted imaging followed by IVIM DW-MRI on a 3.0T scanner (Ingenia, Philips Healthcare, Netherlands) using 20 channel neurovascular phased-array coil. The IVIM DW-MRI images were acquired using a single-shot spin echo planar imaging (SS-EPI) sequence with TR=4000 ms, TE=80-100 ms, FOV=20-24 cm, matrix=128×128, slices=8-10,slicethickness=5mm, NEX=2 and b=0,20,50,80,200,300,500,800,1500,2000 s/mm2. IVIM DW-MRI data analysis: The data were fitted using (a) mono-exponential model to calculate the apparent diffusion coefficient (ADC) and (b) bi-exponential model (IVIM DW-MRI) to estimate the diffusion coefficient (D), perfusion fraction (f) and pseudo diffusion coefficient (D*) (7, 8). Regions of Interest (ROIs) were delineated on the neck nodal metastases by a team of radiation oncologists (NR; >5 years of imaging experience) and a neuroradiologist (VH; >10 years of imaging experience), on the IVIM DW-MRI image (b = 0 s/mm2) with the Eclipse treatment planning system (Varian Medical Systems). The total tumor volume was calculated from T2w images. Statistical analysis: An unsupervised hierarchical clustering (HC) (9-11) was performed with a distance measure based on the Pearson correlation coefficient using an R package gplots (12) to generate a heat map that aims to identify subtypes in HNSCC patients. HC used the relative percentage changes derived from the IVIM imaging metrics during treatment, which resulted in a total of nine sets of values for these imaging metrics. Response was assessed using RECISTv1.1(13).Analysis was performed using Wilcoxon-rank sum test. Bonferroni correction was applied to address the multiple comparisons in ADC, IVIM metrics and total tumor volume. A p-value <0.05 and an adjusted p-value <0.003(0.05/15) were considered significant before and after Bonferroni correction.Results
HNSCC patients in the complete response (CR) group showed an increasing trend for ADC and D at each intra-TX week when compared to pre-TX in the CR group (p<0.003) (Table 1). Figure 1 shows the mean relative (r) percentage changes (mean±std) in ADC (rADCiwk-0wk) (a) and D (rDiwk-0wk) (b), respectively, during the 3 treatment weeks for the patient groups who experienced CR and non-CR. The unsupervised hierarchical clustering demonstrated the existence of clusters in HPV+ patients in the heat map (Figure 1c). It is interesting to note that all the 4 non-CR patients belonged to the same cluster. Additionally, the heat map indicated the possibility of the existence of subtypes in HPV+ HNSCC patients. Figure 2 illustrates MR images, pre- and intra-TX week 3, with overlaid ADC and D parametric maps from a patient (52 years, male) who showed CR. Figures 3 (a) and (b) show the histogram distributions of the pre- and intra-TX week 3 measures of ADC and D for the CR and non-CR patients.Discussion
The CR group rD3w-0wk was significantly higher than in the non-CR group. A hierarchical clustering approach, shown on a heat map, using IVIM DW-MRI, identified subtypes in HPV+ HNSCC patients.Conclusion
After appropriate validation in a larger HNSCC population, these findings may be useful in individualized patient care.Acknowledgements
This work was supported by the MSKCC internal IMRAS grant and in part through the NIH/NCI Cancer Center Support Grant: P30 CA008748.References
1. Psyrri A, Rampias T, Vermorken JB. The current and future impact of human papillomavirus on treatment of squamous cell carcinoma of the head and neck. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25(11):2101-15. doi: 10.1093/annonc/mdu265. PubMed PMID: 25057165. 2. Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9-22. doi: 10.1038/nrc2982. PubMed PMID: WOS:000285575600009. 3. O'Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, Weinreb I, Kim J, Ringash J, Bayley A, Dawson LA, Hope A, Cho J, Irish J, Gilbert R, Gullane P, Hui A, Liu FF, Chen E, Xu W. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(5):543-50. doi: 10.1200/JCO.2012.44.0164. PubMed PMID: 23295795. 4. Huang SH, Perez-Ordonez B, Weinreb I, Hope A, Massey C, Waldron JN, Kim J, Bayley AJ, Cummings B, Cho BC, Ringash J, Dawson LA, Siu LL, Chen E, Irish J, Gullane P, Hui A, Liu FF, Shen X, Xu W, O'Sullivan B. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral oncology. 2013;49(1):79-85. doi: 10.1016/j.oraloncology.2012.07.015. PubMed PMID: 22917550. 5. Kim S, Loevner LA, Quon H, Kilger A, Sherman E, Weinstein G, Chalian A, Poptani H. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR American journal of neuroradiology. 2010;31(2):262-8. doi: 10.3174/ajnr.A1817. PubMed PMID: 19797785. 6. Vandecaveye V, Dirix P, De Keyzer F, Op de Beeck K, Vander Poorten V, Hauben E, Lambrecht M, Nuyts S, Hermans R. Diffusion-weighted magnetic resonance imaging early after chemoradiotherapy to monitor treatment response in head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(3):1098-107. doi: 10.1016/j.ijrobp.2011.02.044. PubMed PMID: 21514067. 7. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401-7. doi: 10.1148/radiology.161.2.3763909. PubMed PMID: 3763909. 8. Lu Y, Jansen JF, Mazaheri Y, Stambuk HE, Koutcher JA, Shukla-Dave A. Extension of the intravoxel incoherent motion model to non-gaussian diffusion in head and neck cancer. Journal of magnetic resonance imaging : JMRI. 2012;36(5):1088-96. doi: 10.1002/jmri.23770. PubMed PMID: 22826198; PubMed Central PMCID: PMC3482143. 9. Ashraf AB, Daye D, Gavenonis S, Mies C, Feldman M, Rosen M, Kontos D. Identification of intrinsic imaging phenotypes for breast cancer tumors: preliminary associations with gene expression profiles. Radiology. 2014;272(2):374-84. doi: 10.1148/radiol.14131375. PubMed PMID: 24702725; PubMed Central PMCID: PMC4564060. 10. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research N. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010;17(1):98-110. doi: 10.1016/j.ccr.2009.12.020. PubMed PMID: 20129251; PubMed Central PMCID: PMC2818769. 11. Zhang F, Chen JY. Breast cancer subtyping from plasma proteins. BMC medical genomics. 2013;6 Suppl 1:S6. doi: 10.1186/1755-8794-6-S1-S6. PubMed PMID: 23369492; PubMed Central PMCID: PMC3552699. 12. R Core Team (2014). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.: Retrieved from: http://www.R-project.org. 13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer. 2009;45(2):228-47. doi: 10.1016/j.ejca.2008.10.026. PubMed PMID: 19097774.Figures
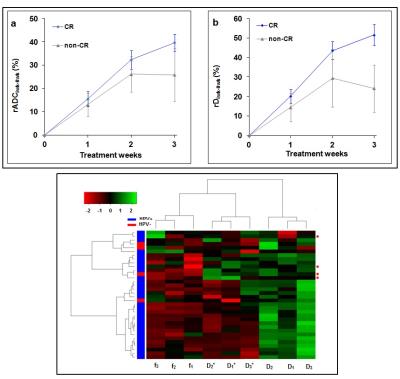
Figure 1. A line graph of the mean (rADCiwk-0wk
(%) (a) and rDiwk-0wk (%) (b) during treatment weeks for the CR and
non-CR groups. Error bars represent the standard error of the mean. rD3wk-0wk
was significantly higher in the CR group compared with the non-CR group
(p=0.007), whereas rADC3wk-0wk did not show (p>0.05). A heat map generated from an unsupervised hierarchical
clustering using the relative percentage change of f(1,2,3), D(1,2,3)
and D*(1,2,3), where 1, 2, 3 represent weeks 1, 2 and 3,
respectively (c). The red circles on the right indicate patients with
non-CR.
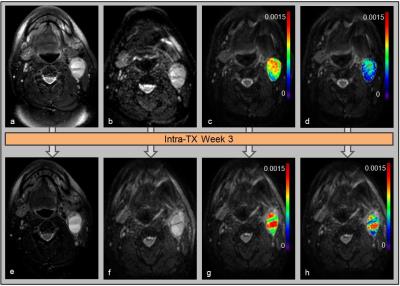
Figure
2. Representative pre-TX (top) and
intra-TX week 3 (bottom) MR images of a patient who showed CR (52 years, male).
T2-weighted images (a and e). Diffusion-weighted (b = 0 s/mm2)
images (b and f). ADC (mm2/s) (c and g) and D (mm2/s) (d
and h) map overlaid on DW (b= 0 s/mm2) images.
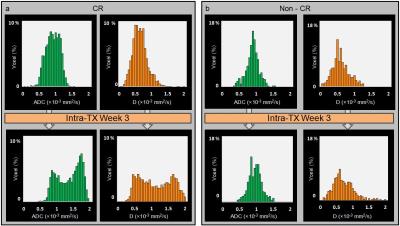
Figure 3:
Representative histogram distributions of the pre- and intra-TX week 3 measurements
of ADC and D, within the ROIs of the metastatic neck node, for the CR (a) and
non-CR (b) patients. Histograms represent the voxel distribution (%) of pre-
and intra-TX week3 ADC (mm2/s) and D (mm2/s) in the CR
and non-CR groups. A shift of
ADC and D values towards a higher value at intra-TX week 3 in the CR group can
be appreciated, whereas in the non-CR group no major changes in ADC and D
values were observed.
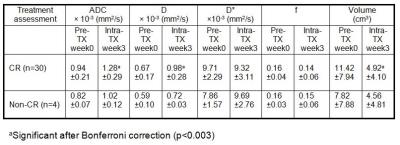
Table1: Pre-TX (week
0) and intra-TX (week 3) IVIM DW-MRI metric values and total tumor volume (mean±std)
from neck nodal metastases of CR and non-CR HPV HNSCC patients.