1115
Hyperpolarized 13C Dynamic Breath-held Molecular Imaging to Detect Targeted Therapy Response in Patients with Liver Metastases1Department of Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2UC Berkeley-UCSF Graduate Program in Bioengineering, UC Berkeley and UCSF, San Francisco, CA, United States, 3University of Cambridge, United Kingdom, 4Department of Clinical Pharmacy, UCSF, San Francisco, CA, United States, 5Department of Medicine, UCSF, San Francisco, CA, United States
Synopsis
New clinical trials are using hyperpolarized 13C molecular imaging technology to evaluate tumor metabolic activity and response to targeted drug therapies. The goal of this work was to develop and apply an experimental setup for HP 13C MR investigations of cancer metastases to liver. In this study, patients with liver metastases were imaged and the results demonstrated sufficient SNR and data quality for the quantification of the localized conversion rate of [1-13C]pyruvate to [1-13C]lactate through lactate dehydrogenase (LDH), which is a pathway targeted by numerous emerging pharmaceutical agents and currently prescribed Everolimus.
Purpose
There is an unmet clinical need for non-invasive imaging to provide early evidence of drug targeting effectiveness, which is crucial for patients with progressing liver metastases who require timely assessment of their treatment. Prior hyperpolarized 13C MR human prostate cancer studies demonstrated safety and feasibility of detecting in vivo tumor metabolism with excellent signal-to-noise ratio (SNR) and valuable enzymatic activity information1, 2, 3. The goal of this study was to translate this technique to human liver imaging, optimize coil and sequence performance, and establish a robust method for quantifying enzymatic kinetics.Methods
Patients with metastases to the liver were imaged in a whole-body 3.0T MRI system using a standard clinical liver imaging protocol and a dynamic 13C echo-planar spectroscopic imaging (EPSI) sequence. [1-13C]Pyruvic acid mixed with trityl radical was pre-filled in fluid paths and polarized in the GE SpinLab system (5T, 0.77K) for approximately 2 hours, yielding 30% polarization in the liquid state. An integrated quality control system analyzed the solution for pH, temperature, concentration, and polarization level right after the dissolution process, and 0.43mL/kg of the dissolved pyruvate solution (250 mM) was injected into the patient by an I.V. infusion pump at a rate of 5mL/sec and followed by a 20mL saline flush. Imaging started 5 seconds after end of the saline flush, and patients were instructed to hold their breath at end of exhalation for as long as possible, and then to resume normal breathing for the rest of the scan.
Proton imaging was performed using body coil transmit and a 16-channel flexible surface coil array. A custom 13C figure-8 transceiver coil was built for carbon signal acquisition covering the liver metastases and nearby normal liver tissue. A 13C-enriched urea sample and vitamin E capsules were placed inside the carbon coil housing to enable accurate coil positioning, power and frequency calibration. Power calibration was first performed using the embedded urea sample, and then adjusted based on a previously acquired phantom B1 map. The B1 map was acquired using the double-angle method.
2D dynamic 13C EPSI data were acquired from a 30-40 mm axial slice with the following key parameters: multiband RF excitation with 10 on pyruvate and 20 on lactate, 3 second temporal resolution with 20 total time frames, 16 phase encodes in the RL direction and a symmetric EPSI readout in the AP direction (12×12mm in-plane resolution) 4.
Coil and sequence performance were evaluated prior to patient studies, by using a large phantom containing ethylene glycol and by detecting natural abundance 13C fat signal from the abdomen of healthy volunteers.
Results and Discussion
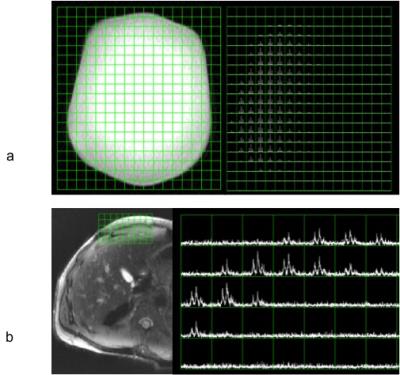
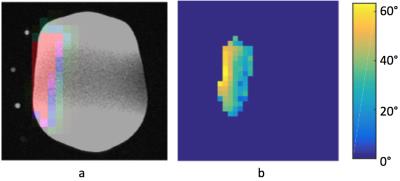
From the ethylene glycol phantom results, the 13C figure-8 coil demonstrated sufficient metabolite signal and a penetration depth about 7-8 cm, as shown in Figure 1a. Natural abundance 13C fat signal was successfully detected from a healthy volunteer as shown in Figure 1b. Figure 2a shows a carbon image overlaid on the proton T1 weighted image from a large ethylene glycol phantom. The 13C-urea sample and vitamin E capsules show up on the T1 weighted image as bright dots to mark the carbon coil position. Figure 2b shows an axial B1 map of the phantom acquired using the double-angle method, which was later used for RF power calibration of the hyperpolarized acquisition.
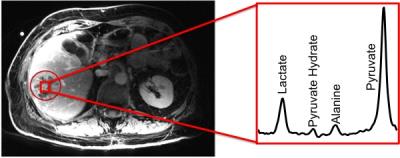
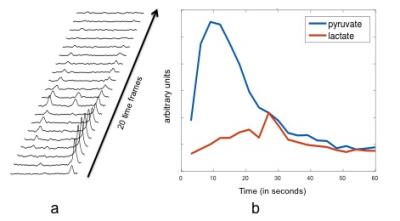
High metabolite SNR was observed from the patient studies. Maximum pyruvate and lactate SNR from the tumor voxels were 70 and 32, respectively. Figure 3 shows a representative patient liver proton image as well as a summed spectrum from a voxel in the tumor. Figure 4 shows the dynamic time course of the tumor spectra. Pyruvate signal peaked at around 9-12 seconds after start of imaging, and lactate signal reached its maximum around 27 seconds after start of imaging.
Conclusions
An experimental strategy using a specialized transceiver carbon coil was successfully developed and applied to phantom and human studies to detect hyperpolarized 13C substrates in liver metastases. The patient studies demonstrated the feasibility of using hyperpolarized 13C pyruvate to investigate the metabolism of liver metastases in vivo in a key metabolic pathway targeted by new therapeutics. These results show great potential for this technique to be applied in clinical trials of new drugs for treating advanced cancer patients including breast, renal cell carcinoma, and endometrial cancer metastases.Acknowledgements
The authors would like to thank Mary McPolin and Patrick Koon for their assistance during the imaging process. This work was supported by NIH grants R01CA183071 and P41EB013598.References
[1] Nelson, Sarah J., et al. "Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate." Science translational medicine 5.198 (2013): 198ra108-198ra108.
[2] Chen, Hsin-Yu, et al. " 3D Dynamic Hyperpolarized 13C-Pyruvate MR Metabolic Imaging of Human Prostate Cancer. " International Society for Magnetic Resonance in Medicine Conference, Singapore, 2016.
[3] Park, Ilwoo, et al. "Hyperpolarized 13C Metabolic Imaging of Patients with Brain Tumors." World Molecular Imaging Conference, New York, US, 2016.
[4] Larson, Peder EZ, et al. "Investigation of tumor hyperpolarized [1-13C]-pyruvate dynamics using time-resolved multiband RF excitation echo-planar MRSI." Magnetic resonance in medicine 63.3 (2010): 582-591.
Figures